Unraveling why we sleep: Quantitative analysis reveals abrupt transition from neural reorganization to repair in early development
- PMID: 32948580
- PMCID: PMC7500925
- DOI: 10.1126/sciadv.aba0398
Unraveling why we sleep: Quantitative analysis reveals abrupt transition from neural reorganization to repair in early development
Abstract
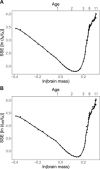
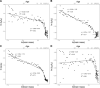
Sleep serves disparate functions, most notably neural repair, metabolite clearance and circuit reorganization. Yet the relative importance remains hotly debated. Here, we create a novel mechanistic framework for understanding and predicting how sleep changes during ontogeny and across phylogeny. We use this theory to quantitatively distinguish between sleep used for neural reorganization versus repair. Our findings reveal an abrupt transition, between 2 and 3 years of age in humans. Specifically, our results show that differences in sleep across phylogeny and during late ontogeny (after 2 or 3 years in humans) are primarily due to sleep functioning for repair or clearance, while changes in sleep during early ontogeny (before 2 or 3 years) primarily support neural reorganization and learning. Moreover, our analysis shows that neuroplastic reorganization occurs primarily in REM sleep but not in NREM. This developmental transition suggests a complex interplay between developmental and evolutionary constraints on sleep.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures