Artificial cells drive neural differentiation
- PMID: 32948587
- PMCID: PMC7500934
- DOI: 10.1126/sciadv.abb4920
Artificial cells drive neural differentiation
Abstract
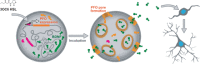
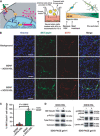
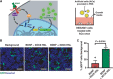
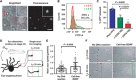
We report the construction of artificial cells that chemically communicate with mammalian cells under physiological conditions. The artificial cells respond to the presence of a small molecule in the environment by synthesizing and releasing a potent protein signal, brain-derived neurotrophic factor. Genetically controlled artificial cells communicate with engineered human embryonic kidney cells and murine neural stem cells. The data suggest that artificial cells are a versatile chassis for the in situ synthesis and on-demand release of chemical signals that elicit desired phenotypic changes of eukaryotic cells, including neuronal differentiation. In the future, artificial cells could be engineered to go beyond the capabilities of typical smart drug delivery vehicles by synthesizing and delivering specific therapeutic molecules tailored to distinct physiological conditions.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures
References
-
- Schwille P., Spatz J., Landfester K., Bodenschatz E., Herminghaus S., Sourjik V., Erb T. J., Bastiaens P., Lipowsky R., Hyman A., Dabrock P., Baret J.-C., Vidakovic-Koch T., Bieling P., Dimova R., Mutschler H., Robinson T., Tang T.-Y. D., Wegner S., Sundmacher K., MaxSynBio: Avenues towards creating cells from the bottom up. Angew. Chem. Int. Ed. Engl. 57, 13382–13392 (2018). - PubMed
-
- Deng N.-N., Yelleswarapu M., Zheng L., Huck W. T. S., Microfluidic assembly of monodisperse vesosomes as artificial cell models. J. Am. Chem. Soc. 139, 587–590 (2017). - PubMed
-
- Elani Y., Law R. V., Ces O., Vesicle-based artificial cells as chemical microreactors with spatially segregated reaction pathways. Nat. Commun. 5, 5305 (2014). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

