Chylomicronemia from GPIHBP1 autoantibodies
- PMID: 32948662
- PMCID: PMC7604722
- DOI: 10.1194/jlr.R120001116
Chylomicronemia from GPIHBP1 autoantibodies
Abstract
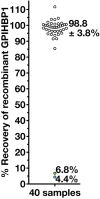
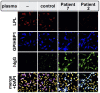
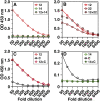
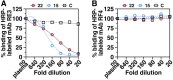
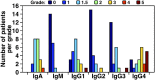
Some cases of chylomicronemia are caused by autoantibodies against glycosylphosphatidylinositol-anchored HDL binding protein 1 (GPIHBP1), an endothelial cell protein that shuttles LPL to the capillary lumen. GPIHBP1 autoantibodies prevent binding and transport of LPL by GPIHBP1, thereby disrupting the lipolytic processing of triglyceride-rich lipoproteins. Here, we review the "GPIHBP1 autoantibody syndrome" and summarize clinical and laboratory findings in 22 patients. All patients had GPIHBP1 autoantibodies and chylomicronemia, but we did not find a correlation between triglyceride levels and autoantibody levels. Many of the patients had a history of pancreatitis, and most had clinical and/or serological evidence of autoimmune disease. IgA autoantibodies were present in all patients, and IgG4 autoantibodies were present in 19 of 22 patients. Patients with GPIHBP1 autoantibodies had low plasma LPL levels, consistent with impaired delivery of LPL into capillaries. Plasma levels of GPIHBP1, measured with a monoclonal antibody-based ELISA, were very low in 17 patients, reflecting the inability of the ELISA to detect GPIHBP1 in the presence of autoantibodies (immunoassay interference). However, GPIHBP1 levels were very high in five patients, indicating little capacity of their autoantibodies to interfere with the ELISA. Recently, several GPIHBP1 autoantibody syndrome patients were treated successfully with rituximab, resulting in the disappearance of GPIHBP1 autoantibodies and normalization of both plasma triglyceride and LPL levels. The GPIHBP1 autoantibody syndrome should be considered in any patient with newly acquired and unexplained chylomicronemia.
Keywords: autoimmune disease; glycosylphosphatidylinositol-anchored high density lipoprotein binding protein 1; immunoglobulin A; immunoglobulin G4; lipoprotein lipase; triglycerides.
Copyright © 2020 Miyashita et al.
Conflict of interest statement
Conflict of interest—K,N. holds stock in Immuno-Biological Laboratories (IBL) and serves as a consultant for Skylight and Sysmex. All other authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

