Pressure overload by suprarenal aortic constriction in mice leads to left ventricular hypertrophy without c-Kit expression in cardiomyocytes
- PMID: 32948799
- PMCID: PMC7501855
- DOI: 10.1038/s41598-020-72273-3
Pressure overload by suprarenal aortic constriction in mice leads to left ventricular hypertrophy without c-Kit expression in cardiomyocytes
Abstract
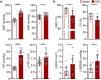
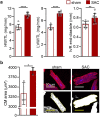
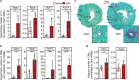
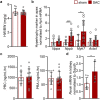
Animal models of pressure overload are valuable for understanding hypertensive heart disease. We characterised a surgical model of pressure overload-induced hypertrophy in C57BL/6J mice produced by suprarenal aortic constriction (SAC). Compared to sham controls, at one week post-SAC systolic blood pressure was significantly elevated and left ventricular (LV) hypertrophy was evident by a 50% increase in the LV weight-to-tibia length ratio due to cardiomyocyte hypertrophy. As a result, LV end-diastolic wall thickness-to-chamber radius (h/R) ratio increased, consistent with the development of concentric hypertrophy. LV wall thickening was not sufficient to normalise LV wall stress, which also increased, resulting in LV systolic dysfunction with reductions in ejection fraction and fractional shortening, but no evidence of heart failure. Pathological LV remodelling was evident by the re-expression of fetal genes and coronary artery perivascular fibrosis, with ischaemia indicated by enhanced cardiomyocyte Hif1a expression. The expression of stem cell factor receptor, c-Kit, was low basally in cardiomyocytes and did not change following the development of robust hypertrophy, suggesting there is no role for cardiomyocyte c-Kit signalling in pathological LV remodelling following pressure overload.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Rats are protected from the stress of chronic pressure overload compared with mice.Am J Physiol Regul Integr Comp Physiol. 2020 May 1;318(5):R894-R900. doi: 10.1152/ajpregu.00370.2019. Epub 2020 Mar 25. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32209023 Free PMC article.
-
Disruption of actin dynamics regulated by Rho effector mDia1 attenuates pressure overload-induced cardiac hypertrophic responses and exacerbates dysfunction.Cardiovasc Res. 2021 Mar 21;117(4):1103-1117. doi: 10.1093/cvr/cvaa206. Cardiovasc Res. 2021. PMID: 32647865
-
Concentric left ventricular remodeling in endothelial nitric oxide synthase knockout mice by chronic pressure overload.Cardiovasc Res. 2005 Jun 1;66(3):444-53. doi: 10.1016/j.cardiores.2005.01.021. Epub 2005 Feb 24. Cardiovasc Res. 2005. PMID: 15914109
-
Large animal models of pressure overload-induced cardiac left ventricular hypertrophy to study remodelling of the human heart with aortic stenosis.Cardiovasc Res. 2024 Apr 30;120(5):461-475. doi: 10.1093/cvr/cvae045. Cardiovasc Res. 2024. PMID: 38428029 Free PMC article. Review.
-
Hypertension and Organ Damage in Women.High Blood Press Cardiovasc Prev. 2018 Sep;25(3):245-252. doi: 10.1007/s40292-018-0265-0. Epub 2018 Jun 26. High Blood Press Cardiovasc Prev. 2018. PMID: 29943358 Review.
Cited by
-
Cardiac Gq Receptors and Calcineurin Activation Are Not Required for the Hypertrophic Response to Mechanical Left Ventricular Pressure Overload.Front Cell Dev Biol. 2021 Feb 15;9:639509. doi: 10.3389/fcell.2021.639509. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33659256 Free PMC article.
-
Anesthesia and analgesia for common research models of adult mice.Lab Anim Res. 2022 Dec 13;38(1):40. doi: 10.1186/s42826-022-00150-3. Lab Anim Res. 2022. PMID: 36514128 Free PMC article. Review.
-
The Ca2+-activated cation channel TRPM4 is a positive regulator of pressure overload-induced cardiac hypertrophy.Elife. 2021 Jun 30;10:e66582. doi: 10.7554/eLife.66582. Elife. 2021. PMID: 34190686 Free PMC article.
-
Calpains as Potential Therapeutic Targets for Myocardial Hypertrophy.Int J Mol Sci. 2022 Apr 7;23(8):4103. doi: 10.3390/ijms23084103. Int J Mol Sci. 2022. PMID: 35456920 Free PMC article. Review.
-
Mechanisms shared between cancer, heart failure, and targeted anti-cancer therapies.Cardiovasc Res. 2023 Feb 3;118(18):3451-3466. doi: 10.1093/cvr/cvac132. Cardiovasc Res. 2023. PMID: 36004495 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

