Analysis of temporal correlation in heart rate variability through maximum entropy principle in a minimal pairwise glassy model
- PMID: 32948805
- PMCID: PMC7501304
- DOI: 10.1038/s41598-020-72183-4
Analysis of temporal correlation in heart rate variability through maximum entropy principle in a minimal pairwise glassy model
Abstract
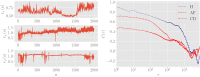
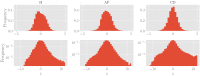
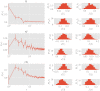
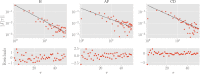
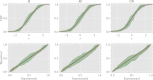
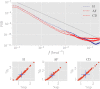
In this work we apply statistical mechanics tools to infer cardiac pathologies over a sample of M patients whose heart rate variability has been recorded via 24 h Holter device and that are divided in different classes according to their clinical status (providing a repository of labelled data). Considering the set of inter-beat interval sequences [Formula: see text], with [Formula: see text], we estimate their probability distribution [Formula: see text] exploiting the maximum entropy principle. By setting constraints on the first and on the second moment we obtain an effective pairwise [Formula: see text] model, whose parameters are shown to depend on the clinical status of the patient. In order to check this framework, we generate synthetic data from our model and we show that their distribution is in excellent agreement with the one obtained from experimental data. Further, our model can be related to a one-dimensional spin-glass with quenched long-range couplings decaying with the spin-spin distance as a power-law. This allows us to speculate that the 1/f noise typical of heart-rate variability may stem from the interplay between the parasympathetic and orthosympathetic systems.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Barra, O.A. & Moretti, L., The “Life Potential” a new complex algorithm to assess “heart rate variability” from Holter records for cognitive and dignostic aims, avaiable atarXiv:1310.7230, (2013).
-
- Jaynes ET. Probability theory: The logic of science. Cambridge: Cambridge University Press; 2003.
-
- Tkacik G, et al. The simplest maximum entropy model for collective behavior in a neural network. JSTAT. 2013;2013(03):3011–3043.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

