Toxic iron species in lower-risk myelodysplastic syndrome patients: course of disease and effects on outcome
- PMID: 32948844
- PMCID: PMC8179850
- DOI: 10.1038/s41375-020-01022-2
Toxic iron species in lower-risk myelodysplastic syndrome patients: course of disease and effects on outcome
Conflict of interest statement
CvM: project manager of the EUMDS Registry, is funded by the EUMDS and MDS-RIGHT project budget; ASm: research funding from Novartis, Cilag-Janssen, and Boehringer Ingelheim; ASy: honoraria and consulting fees from Amgen, Celgene/GenesisPharma, Genzyme/Sanofi, Gilead, Janssen-Cilag, Pfizer, MSD, and Novartis; HG: honoraria from Celgene, Novartis, and Alexion; SK: honoraria from Novartis, Jazz, and Celgene; EH-L: research funding from Celgene; NB: research funding from Novartis, Bristol Meyer Squibb, Pfizer, Ariad, MSD, Astellas, Xenikos, and Celgene, educational grant from Novartis, Celgene, and Janssen-Cilag; DWS: paid employee of RadboudUMC, which offers hepcidin measurements via Hepcidinanalysis.com at a fee for service basis; TdW: research funding from Amgen, Celgene, and Novartis, as project coordinator EUMDS. The other authors declare that they have no conflict of interest.
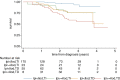
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical