Increased FOXL2 expression alters uterine structures and functions†
- PMID: 32948877
- PMCID: PMC7609875
- DOI: 10.1093/biolre/ioaa143
Increased FOXL2 expression alters uterine structures and functions†
Abstract
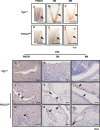
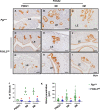
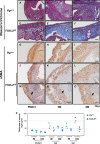
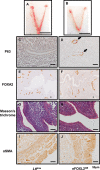
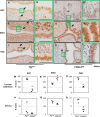
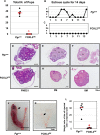
The transcription factor forkhead box L2 (FOXL2) regulates sex differentiation and reproductive function. Elevated levels of this transcription factor have been observed in the diseases of the uterus, such as endometriosis. However, the impact of elevated FOXL2 expression on uterine physiology remains unknown. In order to determine the consequences of altered FOXL2 in the female reproductive axis, we generated mice with over-expression of FOXL2 (FOXL2OE) by crossing Foxl2LsL/+ with the Progesterone receptor Pgrcre model. FOXL2OE uterus showed severe morphological abnormality including abnormal epithelial stratification, blunted adenogenesis, increased endometrial fibrosis, and disrupted myometrial morphology. In contrast, increasing FOXL2 levels specifically in uterine epithelium by crossing the Foxl2LsL/+ with the lactoferrin Ltficre mice resulted in the eFOXL2OE mice with uterine epithelial stratification but without defects in endometrial fibrosis and adenogenesis, demonstrating a role of the endometrial stroma in the uterine abnormalities of the FOXL2OE mice. Transcriptomic analysis of 12 weeks old Pgrcre and FOXL2OE uterus at diestrus stage showed multiple signaling pathways related with cellular matrix, wnt/β-catenin, and altered cell cycle. Furthermore, we found FOXL2OE mice were sterile. The infertility was caused in part by a disruption of the hypophyseal ovarian axis resulting in an anovulatory phenotype. The FOXL2OE mice failed to show decidual responses during artificial decidualization in ovariectomized mice demonstrating the uterine contribution to the infertility phenotype. These data support that aberrantly increased FOXL2 expressions in the female reproductive tract can disrupt ovarian and uterine functions.
Keywords: FOXL2; adenogenesis; fibrosis; myometrial; stratification.
Published by Oxford University Press on behalf of Society for the Study of Reproduction 2020.
Figures

References
-
- Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. FOXL2 in the pituitary: Molecular, genetic, and developmental analysis. Mol Endocrinol 2006; 20:2796–2805. - PubMed
-
- Egashira N, Takekoshi S, Takei M, Teramoto A, Osamura RY. Expression of FOXL2 in human normal pituitaries and pituitary adenomas. Mod Pathol 2011; 24:765–773. - PubMed
-
- Governini L, Carrarelli P, Rocha AL, Leo VD, Luddi A, Arcuri F, Piomboni P, Chapron C, Bilezikjian LM, Petraglia F. FOXL2 in human endometrium: Hyperexpressed in endometriosis. Reprod Sci 2014; 21:1249–1255. - PubMed
-
- Brody JR, Histologic CGR. Morphometric, and Immunocytochemical analysis of Myometrial development in rats and mice. 1. Normal Development. American Journal of Anatomy 1989; 186:1–20. - PubMed
-
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 2004; 131:933–942. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

