Transthyretin Amyloidosis: Update on the Clinical Spectrum, Pathogenesis, and Disease-Modifying Therapies
- PMID: 32948978
- PMCID: PMC7500251
- DOI: 10.1007/s40120-020-00210-7
Transthyretin Amyloidosis: Update on the Clinical Spectrum, Pathogenesis, and Disease-Modifying Therapies
Abstract
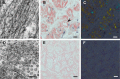
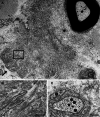
ATTR amyloidosis is caused by systemic deposition of transthyretin (TTR) and comprises ATTRwt (wt for wild-type) amyloidosis, ATTRv (v for variant) amyloidosis, and acquired ATTR amyloidosis after domino liver transplantation. ATTRwt amyloidosis has classically been regarded as cardiomyopathy found in the elderly, whereas carpal tunnel syndrome has also become a major initial manifestation. The phenotypes of ATTRv amyloidosis are diverse and include neuropathy, cardiomyopathy, and oculoleptomeningeal involvement as the predominant features, depending on the mutation and age of onset. In addition to variant TTR, the deposition of wild-type TTR plays a significant role, even in patients with ATTRv amyloidosis. The formation of amyloid fibrils tends to occur in association with the basement membrane. The thickening or reduplication of the basement membrane surrounding endoneurial microvessels, which is similar to diabetic neuropathy, is observed in ATTRv amyloidosis, suggesting that common mechanisms, such as an accumulation of advanced glycation end products, may participate in the disease process. In addition to direct damage caused by amyloid fibrils, recent studies have suggested that the toxicity of nonfibrillar TTRs, such as TTR oligomers, participates in the process of tissue damage. Although liver transplantation has been performed for patients with ATTRv amyloidosis since 1990, late-onset patients were not eligible for this treatment. However, as the efficacy of orally administered tafamidis and diflunisal, which stabilize TTR tetramers, was suggested in the early 2010s, such late-onset patients have also become targets for disease-modifying therapies. Additionally, recent studies of small interfering RNA (patisiran) and antisense oligonucleotide (inotersen) therapies have demonstrated the efficacy of these gene-silencing agents. A strategy for monitoring patients that enables the choice of an appropriate treatment from comprehensive and long-term viewpoints should be established. As many patients with ATTR amyloidosis are aged and have heart failure, they are at increased risk of aggravation if they are infected by SARS-CoV2. The optimal interval of evaluation should also be considered, particularly in this COVID-19 era.
Keywords: Angiopathy; Cardiac amyloidosis; Electron microscopy; Microangiopathy; Pathogenesis; Pathology; Protein misfolding disease; Schwann cell; Therapy; Ultrastructure.
Figures



References
-
- Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019;15:387–404. - PubMed
-
- Benson MD, Buxbaum JN, Eisenberg DS, et al. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid. 2018;25:215–219. - PubMed
-
- Stangou AJ, Heaton ND, Rela M, Pepys MB, Hawkins PN, Williams R. Domino hepatic transplantation using the liver from a patient with familial amyloid polyneuropathy. Transplantation. 1998;65:1496–1498. - PubMed
-
- Koike H, Hashimoto R, Tomita M, et al. Diagnosis of sporadic transthyretin Val30Met familial amyloid polyneuropathy: a practical analysis. Amyloid. 2011;18:53–62. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

