Brain reactions to the use of sensorized hand prosthesis in amputees
- PMID: 32949216
- PMCID: PMC7667362
- DOI: 10.1002/brb3.1734
Brain reactions to the use of sensorized hand prosthesis in amputees
Abstract
Objective: We investigated for the first time the presence of chronic changes in the functional organization of sensorimotor brain areas induced by prolonged training with a bidirectional hand prosthesis.
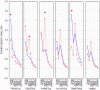
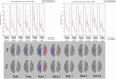
Methods: A multimodal neurophysiological and neuroimaging evaluation of brain functional changes occurring during training in five consecutive amputees participating to experimental trials with robotic hands over a period of 10 years was carried out. In particular, modifications to the functional anatomy of sensorimotor brain areas under resting conditions were explored in order to check for eventual changes with respect to baseline.
Results: Full evidence is provided to demonstrate brain functional changes, and some of them in both the hemispheres and others restricted to the hemisphere contralateral to the amputation/prosthetic hand.
Conclusions: The study describes a unique experimental experience showing that brain reactions to the prolonged use of an artificial hand can be tracked for a tailored approach to a fully embedded artificial upper limb for future chronic uses in daily activities.
Keywords: advanced biotechnologies; brain function; hand prosthesis; personalized medicine.
© 2020 The Authors. Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Caulo, M. , Briganti, C. , Mattei, P. A. , Perfetti, B. , Ferretti, A. , Romani, G. L. , … Colosimo, C. (2007). New morphologic variants of the hand motor cortex as seen with MR imaging in a large study population. AJNR. American Journal of Neuroradiology, 28, 1480–1485. 10.3174/ajnr.A0597 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- FP7-ICT-2013/FET Proactive: Evolving Living Technologies
- 611687/Neurocontrolled Bidirectional Artificial upper limb and hand prosthesis
- Partly funded by the Bertarelli Foundation, the Swiss National Competence Center Research (NCCR) Robotics, and by the CHRONOS project funded by the Swiss National Science Foundation
- Natural sensory feedback for phantom limb pain modulation and therapy
- NEurocontrolled MEchatronic prostheSIS
LinkOut - more resources
Full Text Sources
Medical

