Differential Emergence and Stability of Sensory and Temporal Representations in Context-Specific Hippocampal Sequences
- PMID: 32949502
- PMCID: PMC7736335
- DOI: 10.1016/j.neuron.2020.08.028
Differential Emergence and Stability of Sensory and Temporal Representations in Context-Specific Hippocampal Sequences
Abstract
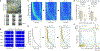
Hippocampal spiking sequences encode external stimuli and spatiotemporal intervals, linking sequential experiences in memory, but the dynamics controlling the emergence and stability of such diverse representations remain unclear. Using two-photon calcium imaging in CA1 while mice performed an olfactory working-memory task, we recorded stimulus-specific sequences of "odor-cells" encoding olfactory stimuli followed by "time-cells" encoding time points in the ensuing delay. Odor-cells were reliably activated and retained stable fields during changes in trial structure and across days. Time-cells exhibited sparse and dynamic fields that remapped in both cases. During task training, but not in untrained task exposure, time-cell ensembles increased in size, whereas odor-cell numbers remained stable. Over days, sequences drifted to new populations with cell activity progressively converging to a field and then diverging from it. Therefore, CA1 employs distinct regimes to encode external cues versus their variable temporal relationships, which may be necessary to construct maps of sequential experiences.
Keywords: CA1; Hippocampus; calcium imaging; drift; learning; odor; population dynamics; sequences; stability; time.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

