Neurological disease in adults with Zika and chikungunya virus infection in Northeast Brazil: a prospective observational study
- PMID: 32949543
- PMCID: PMC7494308
- DOI: 10.1016/S1474-4422(20)30232-5
Neurological disease in adults with Zika and chikungunya virus infection in Northeast Brazil: a prospective observational study
Abstract
Background: Since 2015, the arthropod-borne viruses (arboviruses) Zika and chikungunya have spread across the Americas causing outbreaks, accompanied by increases in immune-mediated and infectious neurological disease. The spectrum of neurological manifestations linked to these viruses, and the importance of dual infection, are not known fully. We aimed to investigate whether neurological presentations differed according to the infecting arbovirus, and whether patients with dual infection had a different disease spectrum or severity.
Methods: We report a prospective observational study done during epidemics of Zika and chikungunya viruses in Recife, Pernambuco, a dengue-endemic area of Brazil. We recruited adults aged 18 years or older referred to Hospital da Restauração, a secondary-level and tertiary-level hospital, with suspected acute neurological disease and a history of suspected arboviral infection. We looked for evidence of Zika, chikungunya, or dengue infection by viral RNA or specific IgM antibodies in serum or CSF. We grouped patients according to their arbovirus laboratory diagnosis and then compared demographic and clinical characteristics.
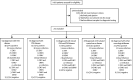
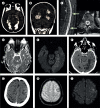
Findings: Between Dec 4, 2014, and Dec 4, 2016, 1410 patients were admitted to the hospital neurology service; 201 (14%) had symptoms consistent with arbovirus infection and sufficient samples for diagnostic testing and were included in the study. The median age was 48 years (IQR 34-60), and 106 (53%) were women. 148 (74%) of 201 patients had laboratory evidence of arboviral infection. 98 (49%) of them had a single viral infection (41 [20%] had Zika, 55 [27%] had chikungunya, and two [1%] had dengue infection), whereas 50 (25%) had evidence of dual infection, mostly with Zika and chikungunya viruses (46 [23%] patients). Patients positive for arbovirus infection presented with a broad range of CNS and peripheral nervous system (PNS) disease. Chikungunya infection was more often associated with CNS disease (26 [47%] of 55 patients with chikungunya infection vs six [15%] of 41 with Zika infection; p=0·0008), especially myelitis (12 [22%] patients). Zika infection was more often associated with PNS disease (26 [63%] of 41 patients with Zika infection vs nine [16%] of 55 with chikungunya infection; p≤0·0001), particularly Guillain-Barré syndrome (25 [61%] patients). Patients with Guillain-Barré syndrome who had Zika and chikungunya dual infection had more aggressive disease, requiring intensive care support and longer hospital stays, than those with mono-infection (median 24 days [IQR 20-30] vs 17 days [10-20]; p=0·0028). Eight (17%) of 46 patients with Zika and chikungunya dual infection had a stroke or transient ischaemic attack, compared with five (6%) of 96 patients with Zika or chikungunya mono-infection (p=0·047).
Interpretation: There is a wide and overlapping spectrum of neurological manifestations caused by Zika or chikungunya mono-infection and by dual infections. The possible increased risk of acute cerebrovascular disease in patients with dual infection merits further investigation.
Funding: Fundação do Amparo a Ciência e Tecnologia de Pernambuco (FACEPE), EU's Horizon 2020 research and innovation programme, National Institute for Health Research.
Translations: For the Portuguese and Spanish translations of the abstract see Supplementary Materials section.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Neurological complications in adults with Zika and chikungunya virus infection.Lancet Neurol. 2020 Oct;19(10):799-801. doi: 10.1016/S1474-4422(20)30309-4. Epub 2020 Sep 16. Lancet Neurol. 2020. PMID: 32949531 No abstract available.
References
-
- Cordeiro MT, Schatzmayr HG, Nogueira RMR, Oliveira VF, Melo WT, Carvalho EF. Dengue and dengue hemorrhagic fever in the State of Pernambuco, 1995–2006. Rev Soc Bras Med Trop. 2007;40:605–611. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

