Ambulatory Reflux Monitoring Guides Proton Pump Inhibitor Discontinuation in Patients With Gastroesophageal Reflux Symptoms: A Clinical Trial
- PMID: 32949568
- PMCID: PMC7755671
- DOI: 10.1053/j.gastro.2020.09.013
Ambulatory Reflux Monitoring Guides Proton Pump Inhibitor Discontinuation in Patients With Gastroesophageal Reflux Symptoms: A Clinical Trial
Abstract
Background and aims: Proton pump inhibitor (PPI) therapy fails to provide adequate symptom control in up to 50% of patients with gastroesophageal reflux symptoms. Although a proportion do not require ongoing PPI therapy, a diagnostic approach to identify candidates appropriate for PPI cessation is not available. This study aimed to examine the clinical utility of prolonged wireless reflux monitoring to predict the ability to discontinue PPIs.
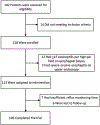
Methods: This double-blinded clinical trial performed over 3 years at 2 centers enrolled adults with troublesome esophageal symptoms of heartburn, regurgitation, and/or chest pain and inadequate PPI response. Participants underwent prolonged wireless reflux monitoring (off PPIs for ≥7 days) and a 3-week PPI cessation intervention. Primary outcome was tolerance of PPI cessation (discontinued or resumed PPIs). Symptom burden was quantified using the Reflux Symptom Questionnaire electronic Diary (RESQ-eD).
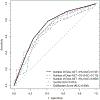
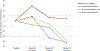
Results: Of 128 enrolled, 100 participants met inclusion criteria (mean age, 48.6 years; 41 men). Thirty-four participants (34%) discontinued PPIs. The strongest predictor of PPI discontinuation was number of days with acid exposure time (AET) > 4.0% (odds ratio, 1.82; P < .001). Participants with 0 days of AET > 4.0% had a 10 times increased odds of discontinuing PPI than participants with 4 days of AET > 4.0%. Reduction in symptom burden was greater among the discontinued versus resumed PPI group (RESQ-eD, -43.7% vs -5.3%; P = .04).
Conclusions: Among patients with typical reflux symptoms, inadequate PPI response, and absence of severe esophagitis, acid exposure on reflux monitoring predicted the ability to discontinue PPIs without symptom escalation. Upfront reflux monitoring off acid suppression can limit unnecessary PPI use and guide personalized management. (ClinicalTrials.gov, Number: NCT03202537).
Keywords: Bravo; Functional Heartburn; Gastroesophageal Reflux Disease (GERD); Wireless pH Monitoring.
Copyright © 2021 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest:
RY: Consultant: Medtronic, Ironwood Pharmaceuticals, Diversatek; Research support: Ironwood Pharmaceuticals; Advisory Board: Phatom Pharmaceuticals
CPG: Consultant: Medtronic, Diversatek, Ironwood, Iso-Thrive, Quintiles
DAC: Consultant: Medtronic
PJK: Research support: Ironwood Pharmaceuticals; Advisory Board: Ironwood Pharmaceuticals
MFV: Consultant: Ironwood Pharmaceuticals, Diversatek, Phathom Pharmaceuticals, Daewood
Patent on mucosal integrity by Vanderbilt
JEP: Consultant: Medtronic, Ironwood Pharmaceuticals, Diversatek; Research support: Ironwood Pharmaceuticals, Takeda; Advisory Board: Medtronic, Diversatek; Stock Options: Crospon Inc
MM, BDN, JT, AJ, LK, AK: None
Figures

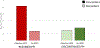
Comment in
-
The Proton Pump Inhibitor Is Not Working: Assess Don't Guess.Gastroenterology. 2021 Jan;160(1):19-20. doi: 10.1053/j.gastro.2020.10.043. Epub 2020 Nov 3. Gastroenterology. 2021. PMID: 33157096 No abstract available.
References
-
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900–20; quiz 1943. - PubMed
-
- Spechler SJ, Hunter JG, Jones KM, et al. Randomized Trial of Medical versus Surgical Treatment for Refractory Heartburn. N Engl J Med 2019;381:1513–1523. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

