A synthetic STING agonist inhibits the replication of human parainfluenza virus 3 and rhinovirus 16 through distinct mechanisms
- PMID: 32949635
- PMCID: PMC7494516
- DOI: 10.1016/j.antiviral.2020.104933
A synthetic STING agonist inhibits the replication of human parainfluenza virus 3 and rhinovirus 16 through distinct mechanisms
Abstract
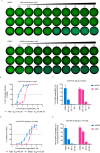
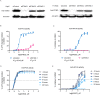
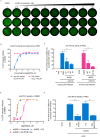
Stimulator of interferon genes (STING), as a signaling hub in innate immunity, plays a central role for the effective initiation of host defense mechanisms against microbial infections. Upon binding of its ligand cyclic dinucleotides (CDNs) produced by the cyclic GMP-AMP synthase (cGAS) or invading bacteria, STING is activated, leading to the induction of both type I interferon responses and autophagy, which are critical for the control of certain microbial infections. RNA viruses, such as Parainfluenza virus (PIV) and Rhinovirus (HRV), are among the leading causes of respiratory infections that affect human health without effective treatments. Activation of STING pathway may provide a new therapeutic approach fighting against these viruses. However, the role of STING in the control of RNA virus infection remains largely unexplored. In this study, using dimeric amidobenzimidazole (diABZI), a newly discovered synthetic small molecule STING receptor agonist with much higher potency than CDNs, we found that activation of STING elicits potent antiviral effects against parainfluenza virus type 3 (PIV3) and human rhinovirus 16 (HRV16), two representative respiratory viral pathogens. Notably, while anti-PIV3 activity was depend on the induction of type I interferon responses through TANK-binding kinase 1 (TBK1), anti-HRV16 activity required the induction of autophagy-related gene 5 (ATG5)-dependent autophagy, indicating that two distinct antiviral mechanisms are engaged upon STING activation. Antiviral activity and individual specific pathway was further confirmed in infected primary bronchial epithelial cells. Our findings thus demonstrate the distinct antiviral mechanisms triggered by STING agonist and uncover the potential of therapeutic effect against different viruses.
Keywords: Autophagy; Parainfluenza virus; Rhinovirus; STING agonist; diABZI.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors are employees of F. Hoffman-La Roche Ltd.
Figures


References
-
- Aguirre S., Maestre A.M., Pagni S., Patel J.R., Savage T., Gutman D., Maringer K., Bernal-Rubio D., Shabman R.S., Simon V., Rodriguez-Madoz J.R., Mulder L.C., Barber G.N., Fernandez-Sesma A. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012;8 - PMC - PubMed
-
- Atreya P.L., Kulkarni S. Respiratory syncytial virus strain A2 is resistant to the antiviral effects of type I interferons and human MxA. Virology. 1999;261:227–241. - PubMed
-
- Bloom B., Cohen R.A., Freeman G. Summary health statistics for U.S. Children: national health interview survey, 2008. Vital Health Stat. 2009;10:1–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

