JNK signalling regulates antioxidant responses in neurons
- PMID: 32949970
- PMCID: PMC7502373
- DOI: 10.1016/j.redox.2020.101712
JNK signalling regulates antioxidant responses in neurons
Abstract
Reactive oxygen species (ROS) are generated during physiological bouts of synaptic activity and as a consequence of pathological conditions in the central nervous system. How neurons respond to and distinguish between ROS in these different contexts is currently unknown. In Drosophila mutants with enhanced JNK activity, lower levels of ROS are observed and these animals are resistant to both changes in ROS and changes in synapse morphology induced by oxidative stress. In wild type flies, disrupting JNK-AP-1 signalling perturbs redox homeostasis suggesting JNK activity positively regulates neuronal antioxidant defense. We validated this hypothesis in mammalian neurons, finding that JNK activity regulates the expression of the antioxidant gene Srxn-1, in a c-Jun dependent manner. We describe a conserved 'adaptive' role for neuronal JNK in the maintenance of redox homeostasis that is relevant to several neurodegenerative diseases.
Keywords: Drosophila; Glutathione; Hydrogen Peroxide; ROS.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



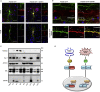


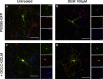
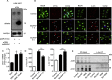
References
-
- Dringen R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000;62:649–671. - PubMed
-
- Milton V.J., Sweeney S.T. Oxidative stress in synapse development and function. Develop. Neurobiol. 2012;72:100–110. - PubMed
-
- Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3:205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

