Dengue and Zika virus infections are enhanced by live attenuated dengue vaccine but not by recombinant DSV4 vaccine candidate in mouse models
- PMID: 32949997
- PMCID: PMC7501058
- DOI: 10.1016/j.ebiom.2020.102991
Dengue and Zika virus infections are enhanced by live attenuated dengue vaccine but not by recombinant DSV4 vaccine candidate in mouse models
Abstract
Background: A tetravalent live attenuated dengue vaccine, Dengvaxia, sensitised naïve recipients to severe dengue illness upon a subsequent natural dengue infection and is suspected to be due to antibody-dependent enhancement (ADE). ADE has also been implicated in the severe neurological outcomes of Zika virus (ZIKV) infection. It has become evident that cross-reactive antibodies targeting the viral pre-membrane protein and fusion-loop epitope are ADE-competent. A pre-clinical tetravalent dengue sub-unit vaccine candidate, DSV4, eliminates these ADE-competent epitopes.
Methods: We compared protective efficacy and ADE-competence of murine polyclonal antibodies induced by DSV4, Dengvaxia and an 'in house' tetravalent mixture of all four laboratory DENV strains, TV DENV, using established mouse models.
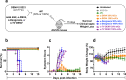
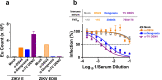
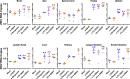
Findings: DSV4-induced antibodies, known to be predominantly type-specific, provided significant protection against lethal DENV challenge, but did not promote ADE of either DENV or ZIKV infection in vivo. Antibodies elicited by Dengvaxia and TV DENV, which are predominantly cross-reactive, not only failed to offer protection against lethal DENV challenge, but also promoted ADE of both DENV and ZIKV infection in vivo.
Interpretation: Protective efficacy against DENV infection may be linked to the induction of neutralising antibodies which are type-specific rather than cross-reactive. Whole virus-based dengue vaccines may be associated with ADE risk, despite their potent virus-neutralising capacity. Vaccines designed to eliminate ADE-competent epitopes may help eliminate/minimise ADE risk.
Funding: This study was supported partly by ICGEB, India, the National Biopharma Mission, DBT, Government of India, Sun Pharmaceutical Industries Limited, India, and NIAID, NIH, USA.
Keywords: AG129; Antibody-dependent enhancement; C57BL/6 Stat2(−/−); DSV4; Dengue VLP vaccine; Dengue virus; Dengvaxia; LAV; Zika virus.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





References
-
- Pierson TC, Diamond MS. Flaviviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Wolters Kluwer and Lippincott Williams & Wilkins; Philadelphia, PA: 2013. pp. 747–794.
-
- Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. - PubMed
-
- Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445–1459. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

