Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks
- PMID: 32950262
- PMCID: PMC7960570
- DOI: 10.1016/j.tibtech.2020.08.007
Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks
Abstract
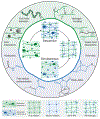
Traditional hydrogels are strong candidates for biomedical applications; however, they may suffer from drawbacks such as weak mechanics, static properties, and an inability to fully replicate aspects of the cellular microenvironment. These challenges can be addressed through the incorporation of second networks to form interpenetrating polymer network (IPN) hydrogels. The objective of this review is to establish clear trends on the enhanced functionality achieved by incorporating secondary networks into traditional, biopolymer-based hydrogels. These include mechanical reinforcement, 'smart' systems that respond to external stimuli, and the ability to tune cell-material interactions. Through attention to network structure and chemistry, IPN hydrogels may advance to meet challenging criteria for a wide range of biomedical fields.
Keywords: biopolymers; cell–material interactions; double networks; interpenetrating network hydrogels; mechanical reinforcement; stimuli-responsive materials.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures




References
-
- Neves SC et al. (2020) Leveling up hydrogels: hybrid systems in tissue engineering. Trends Biotechnol. 38, 292–315 - PubMed
-
- Gong JP (2010) Why are double network hydrogels so tough? Soft Matter 6, 2583–2590
-
- Costa AMS and Mano JF (2015) Extremely strong and tough hydrogels as prospective candidates for tissue repair - a review. Eur. Polym. J 72, 344–364
-
- Higa K et al. (2016) Fundamental biomaterial properties of tough glycosaminoglycan-containing double network hydrogels newly developed using the molecular stent method. Acta Biomater. 43, 38–49 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

