Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development
- PMID: 32950264
- PMCID: PMC7832760
- DOI: 10.1016/j.arcmed.2020.09.010
Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development
Abstract
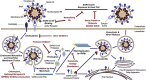
The Coronavirus disease 2019 (COVID-19) pandemic has spread to almost all nooks and corners of the world. There are numerous potential approaches to pharmacologically fight COVID-19: small-molecule drugs, interferon therapies, vaccines, oligonucleotides, peptides, and monoclonal antibodies. Medications are being developed to target the spike, membrane, nucleocapsid or envelope proteins. The spike protein is also a critical target for vaccine development. Immunoinformatic approaches are being used for the identification of B cell and cytotoxic T lymphocyte (CTL) epitopes in the SARS-CoV-2 spike protein. Different vaccine vectors are also being developed. Chemical and physical methods such as formaldehyde, UV light or β-propiolactone are being deployed for the preparation of inactivated virus vaccine. Currently, there are many vaccines undergoing clinical trials. Even though mRNA and DNA vaccines are being designed and moved into clinical trials, these types of vaccines are yet to be approved by regulatory bodies for human use. This review focuses on the drugs and vaccines being developed against the COVID-19.
Keywords: COVID-19; Coronavirus; Drugs; SARS-CoV-2; Spike protein; Vaccine.
Copyright © 2020 IMSS. Published by Elsevier Inc. All rights reserved.
Figures






Comment in
-
Regarding the Article: Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development.Arch Med Res. 2021 May;52(4):456-457. doi: 10.1016/j.arcmed.2020.12.012. Epub 2021 Jan 12. Arch Med Res. 2021. PMID: 33483149 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

