Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons
- PMID: 32950560
- PMCID: PMC8207535
- DOI: 10.1016/j.neuropharm.2020.108296
Sex differences in corticotropin releasing factor peptide regulation of inhibitory control and excitability in central amygdala corticotropin releasing factor receptor 1-neurons
Abstract
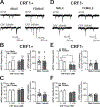
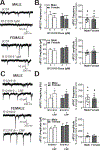
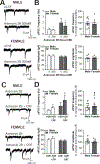
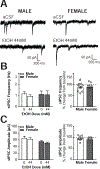
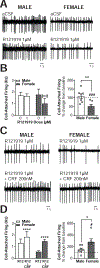
The central amygdala (CeA) is a critical regulator of emotional behavior that has been implicated in psychiatric illnesses, including anxiety disorders and addiction. The CeA corticotropin releasing factor receptor 1 (CRF1) system has been implicated in alcohol use disorder (AUD) and mood disorders, and has been shown to regulate anxiety-like behavior and alcohol consumption in rodents. However, the effects of CRF signaling within the CRF receptor 1-containing (CRF1+) population of the CeA remain unclear, and the effects of ethanol and CRF1 manipulations in female rodents have not been assessed. Here, we characterized inhibitory control and CRF1 signaling in male and female CRF1-GFP reporter mice. Male and female CRF1+ CeA neurons exhibited similar baseline GABAergic signaling and excitability and were comparably sensitive to CRF-induced increases in presynaptic GABA release. CRF1 antagonism reduced GABA release onto CRF1-containing neurons comparably in both males and females. Acute ethanol application reduced GABA release onto CRF1+ neurons from males, but female CRF1+ neurons were insensitive to ethanol. Exogenous CRF increased the firing rate of CRF1-containing neurons to a greater extent in male cells versus female cells, and CRF1 antagonism reduced firing in females but not males. Together, these findings indicate a critical sex-specific role for the CRF system in regulating inhibitory control and excitability of CRF1-containing neurons in the central amygdala. Sex differences in sensitivity of CRF/CRF1 signaling provide useful context for the sex differences in psychiatric illness reported in human patients, particularly AUD.
Keywords: Astressin 2B; CRF; CeA; Female; GABA; R121919.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures


References
-
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:877, 896–904. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

