Collagen XI regulates the acquisition of collagen fibril structure, organization and functional properties in tendon
- PMID: 32950601
- PMCID: PMC7722227
- DOI: 10.1016/j.matbio.2020.09.001
Collagen XI regulates the acquisition of collagen fibril structure, organization and functional properties in tendon
Abstract
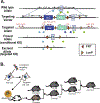
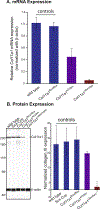
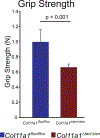
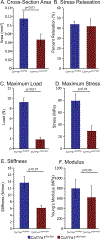
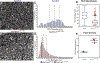
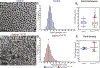
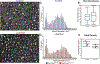
Collagen XI is a fibril-forming collagen that regulates collagen fibrillogenesis. Collagen XI is normally associated with collagen II-containing tissues such as cartilage, but it also is expressed broadly during development in collagen I-containing tissues, including tendons. The goals of this study are to define the roles of collagen XI in regulation of tendon fibrillar structure and the relationship to function. A conditional Col11a1-null mouse model was created to permit the spatial and temporal manipulation of Col11a1 expression. We hypothesize that collagen XI functions to regulate fibril assembly, organization and, therefore, tendon function. Previous work using cho mice with ablated Col11a1 alleles supported roles for collagen XI in tendon fibril assembly. Homozygous cho/cho mice have a perinatal lethal phenotype that limited the studies. To circumvent this, a conditional Col11a1flox/flox mouse model was created where exon 3 was flanked with loxP sites. Breeding with Scleraxis-Cre (Scx-Cre) mice yielded a tendon-specific Col11a1-null mouse line, Col11a1Δten/Δten. Col11a1flox/flox mice had no phenotype compared to wild type C57BL/6 mice and other control mice, e.g., Col11a1flox/flox and Scx-Cre. Col11a1flox/flox mice expressed Col11a1 mRNA at levels comparable to wild type and Scx-Cre mice. In contrast, in Col11a1Δten/Δten mice, Col11a1 mRNA expression decreased to baseline in flexor digitorum longus tendons (FDL). Collagen XI protein expression was absent in Col11a1Δten/Δten FDLs, and at ~50% in Col11a1+/Δten compared to controls. Phenotypically, Col11a1Δten/Δten mice had significantly decreased body weights (p < 0.001), grip strengths (p < 0.001), and with age developed gait impairment becoming hypomobile. In the absence of Col11a1, the tendon collagen fibrillar matrix was abnormal when analyzed using transmission electron microscopy. Reducing Col11a1 and, therefore collagen XI content, resulted in abnormal fibril structure, loss of normal fibril diameter control with a significant shift to small diameters and disrupted parallel alignment of fibrils. These alterations in matrix structure were observed in developing (day 4), maturing (day 30) and mature (day 60) mice. Altering the time of knockdown using inducible I-Col11a1-/- mice indicated that the primary regulatory foci for collagen XI was in development. In mature Col11a1Δten/Δten FDLs a significant decrease in the biomechanical properties was observed. The decrease in maximum stress and modulus suggest that fundamental differences in the material properties in the absence of Col11a1 expression underlie the mechanical deficiencies. These data demonstrate an essential role for collagen XI in regulation of tendon fibril assembly and organization occurring primarily during development.
Keywords: Col11a1; Collagen XI; Collagen fibrillogenesis; Conditional mouse model; Tendon; Tendon biomechanics; Tendon structure.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interests.
Figures


Similar articles
-
Targeted conditional collagen XII deletion alters tendon function.Matrix Biol Plus. 2022 Oct 7;16:100123. doi: 10.1016/j.mbplus.2022.100123. eCollection 2022 Dec. Matrix Biol Plus. 2022. PMID: 36311462 Free PMC article.
-
Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon.J Biol Chem. 2011 Jun 10;286(23):20455-65. doi: 10.1074/jbc.M111.223693. Epub 2011 Apr 5. J Biol Chem. 2011. PMID: 21467034 Free PMC article.
-
Regulatory Role of Collagen XI in the Establishment of Mechanical Properties of Tendons and Ligaments in Mice Is Tissue Dependent.J Biomech Eng. 2025 Jan 1;147(1):011003. doi: 10.1115/1.4066570. J Biomech Eng. 2025. PMID: 39297758
-
Development of tendon structure and function: regulation of collagen fibrillogenesis.J Musculoskelet Neuronal Interact. 2005 Mar;5(1):5-21. J Musculoskelet Neuronal Interact. 2005. PMID: 15788867 Review.
-
Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly.Birth Defects Res C Embryo Today. 2008 Sep;84(3):228-44. doi: 10.1002/bdrc.20130. Birth Defects Res C Embryo Today. 2008. PMID: 18773462 Review.
Cited by
-
Hierarchical ultrastructure: An overview of what is known about tendons and future perspective for tendon engineering.Bioact Mater. 2021 Jun 29;8:124-139. doi: 10.1016/j.bioactmat.2021.06.007. eCollection 2022 Feb. Bioact Mater. 2021. PMID: 34541391 Free PMC article. Review.
-
Characterization of TGFβ1-induced tendon-like structure in the scaffold-free three-dimensional tendon cell culture system.Sci Rep. 2024 Apr 25;14(1):9495. doi: 10.1038/s41598-024-60221-4. Sci Rep. 2024. PMID: 38664570 Free PMC article.
-
Therapeutic Potential of Mesenchymal Stem Cell and Tenocyte Secretomes for Tendon Repair: Proteomic Profiling and Functional Characterization In Vitro and In Ovo.Int J Mol Sci. 2025 Apr 11;26(8):3622. doi: 10.3390/ijms26083622. Int J Mol Sci. 2025. PMID: 40332130 Free PMC article.
-
Mammal comparative tendon biology: advances in regulatory mechanisms through a computational modeling.Front Vet Sci. 2023 Apr 27;10:1175346. doi: 10.3389/fvets.2023.1175346. eCollection 2023. Front Vet Sci. 2023. PMID: 37180059 Free PMC article.
-
Genipin increases extracellular matrix synthesis preventing corneal perforation.Ocul Surf. 2023 Apr;28:115-123. doi: 10.1016/j.jtos.2023.02.003. Epub 2023 Mar 3. Ocul Surf. 2023. PMID: 36871831 Free PMC article.
References
-
- Birk DE, Bruckner P, Collagens, suprastructures and collagen fibril assembly, in: Mecham RP (Ed.), The Extracellular Matrix: an Overview, Springer, NY, 2011, pp. 77–115.
-
- Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE, Development of tendon structure and function: regulation of collagen fibrillogenesis, J. Musculoskelet. Neuronal Interact. 5 (1) (2005) 5–21. - PubMed
-
- Mienaltowski MJ, Birk DE, Structure, physiology, and biochemistry of collagens, Adv. Exp. Med. Biol. 802 (2014) 5–29. - PubMed
-
- Walraven M, Hinz B, Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer, Matrix Biol. 71–72 (2018) 205–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

