Placental Pathology Findings during and after SARS-CoV-2 Infection: Features of Villitis and Malperfusion
- PMID: 32950981
- PMCID: PMC7573905
- DOI: 10.1159/000511324
Placental Pathology Findings during and after SARS-CoV-2 Infection: Features of Villitis and Malperfusion
Abstract
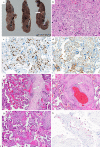
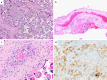
Since the outbreak of coronavirus disease 2019 (COVID-19), there has been a debate whether pregnant women are at a specific risk for COVID-19 and whether it might be vertically transmittable through the placenta. We present a series of five placentas of SARS coronavirus 2 (SARS-CoV-2)-positive women who had been diagnosed with mild symptoms of COVID-19 or had been asymptomatic before birth. We provide a detailed histopathologic description of morphological changes accompanied by an analysis of presence of SARS-CoV-2 in the placental tissue. All placentas were term deliveries (40th and 41st gestational weeks). One SARS-CoV-2-positive patient presented with cough and dyspnoea. This placenta showed prominent lymphohistiocytic villitis and intervillositis and signs of maternal and foetal malperfusion. Viral RNA was present in both placenta tissue and the umbilical cord and could be visualized by in situ hybridization in the decidua. SARS-CoV-2 tests were negative at the time of delivery of 3/5 women, and their placentas did not show increased inflammatory infiltrates. Signs of maternal and/or foetal malperfusion were present in 100% and 40% of cases, respectively. There was no transplacental transmission to the infants. In our cohort, we can document different time points regarding SARS-CoV-2 infection. In acute COVID-19, prominent lymphohistiocytic villitis may occur and might potentially be attributable to SARS-CoV-2 infection of the placenta. Furthermore, there are histopathological signs of maternal and foetal malperfusion, which might have a relationship to an altered coagulative or microangiopathic state induced by SARS-CoV-2, yet this cannot be proven considering a plethora of confounding factors.
Keywords: COVID-19; Chronic villitis; Malperfusion; Placenta.
© 2020 S. Karger AG, Basel.
Conflict of interest statement
The authors declare to have no competing interests.
Figures
References
-
- Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. UK Obstetric Surveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020 Jun;369:m2107. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

