Efficacy and Safety of Tocilizumab for Polyarticular-Course Juvenile Idiopathic Arthritis in the Open-Label Two-Year Extension of a Phase III Trial
- PMID: 32951358
- PMCID: PMC7986602
- DOI: 10.1002/art.41528
Efficacy and Safety of Tocilizumab for Polyarticular-Course Juvenile Idiopathic Arthritis in the Open-Label Two-Year Extension of a Phase III Trial
Abstract
Objective: To report the 2-year efficacy and safety of tocilizumab (TCZ) in patients with polyarticular-course juvenile idiopathic arthritis (JIA).
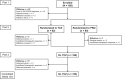
Methods: Patients ages 2-17 years with active polyarticular-course JIA, in whom treatment with methotrexate was unsuccessful, received 16 weeks of open-label intravenous TCZ in part 1 (once every 4 weeks: 8 mg/kg or 10 mg/kg for body weight [BW] <30 kg; 8 mg/kg for BW ≥30 kg). Assessments were based on the JIA-American College of Rheumatology (ACR) response (defined as percentage of improvement in ≥3 of the 6 JIA core response variables [CRVs]). Patients with at least a JIA-ACR30 response (defined as ≥30% improvement in ≥3 of the 6 JIA CRVs without worsening in >1 of the remaining JIA CRVs by >30%) at week 16 were randomly assigned (1:1) to receive TCZ or placebo in part 2. Patients remained in part 2 until either week 40 or the occurrence of JIA flare. Upon starting part 3, all patients received open-label TCZ. At week 104 of the study, efficacy was assessed using JIA-ACR50/70/90 response rates (defined as 50%, 70%, or 90% improvement, respectively), achievement of inactive disease, and the Juvenile Arthritis Disease Activity Score in 71 joints (JADAS-71). Safety was assessed in the all-exposure population per 100 patient-years of exposure.
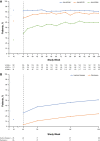
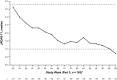
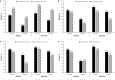
Results: Overall, 188 patients entered part 1, 166 patients entered part 2, and 160 patients entered part 3. By week 104, among the 188 patients in the modified intent-to-treat group who received TCZ, JIA-ACR50/70/90 response rates were 80.3%/77.1%/59.6%, respectively, the median JADAS-71 score decreased from 3.6 at week 40 to 0.7 at week 104, 51.1% of patients had achieved inactive disease, and 31 of 66 patients who had been receiving glucocorticoids discontinued them. Adverse event (AE) and serious AE rates were 406.5 per 100 patient-years and 11.1 per 100 patient-years, respectively. The infection rate was 151.4 per 100 patient-years, and the serious infection rate was 5.2 per 100 patient-years.
Conclusion: Patients treated with TCZ for polyarticular-course JIA showed high-level disease control for up to 2 years. The TCZ safety profile was consistent with that previously reported.
© 2020 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
References
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. - PubMed
-
- Ruperto N, Giannini EH, Pistorio A, Brunner HI, Martini A, Lovell DJ. Is it time to move to active comparator trials in juvenile idiopathic arthritis? A review of current study designs [review]. Arthritis Rheum 2010;62:3131–9. - PubMed
-
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. - PubMed
-
- Zak M, Muller J, Karup PF. Final height, armspan, subischial leg length and body proportions in juvenile chronic arthritis: a long‐term follow‐up study. Horm Res 1999;52:80–5. - PubMed
-
- Lovell DJ. Update on treatment of arthritis in children: new treatments, new goals [review]. Bull NYU Hosp Jt Dis 2006;64:72–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

