Molecular Evolution of Transition Metal Bioavailability at the Host-Pathogen Interface
- PMID: 32951986
- PMCID: PMC7969482
- DOI: 10.1016/j.tim.2020.08.001
Molecular Evolution of Transition Metal Bioavailability at the Host-Pathogen Interface
Abstract
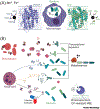
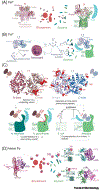
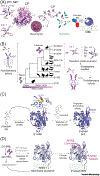
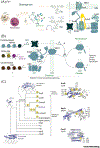
The molecular evolution of the adaptive response at the host-pathogen interface has been frequently referred to as an 'arms race' between the host and bacterial pathogens. The innate immune system employs multiple strategies to starve microbes of metals. Pathogens, in turn, develop successful strategies to maintain access to bioavailable metal ions under conditions of extreme restriction of transition metals, or nutritional immunity. However, the processes by which evolution repurposes or re-engineers host and pathogen proteins to perform or refine new functions have been explored only recently. Here we review the molecular evolution of several human metalloproteins charged with restricting bacterial access to transition metals. These include the transition metal-chelating S100 proteins, natural resistance-associated macrophage protein-1 (NRAMP-1), transferrin, lactoferrin, and heme-binding proteins. We examine their coevolution with bacterial transition metal acquisition systems, involving siderophores and membrane-spanning metal importers, and the biological specificity of allosteric transcriptional regulatory proteins tasked with maintaining bacterial metallostasis. We also discuss the evolution of metallo-β-lactamases; this illustrates how rapid antibiotic-mediated evolution of a zinc metalloenzyme obligatorily occurs in the context of host-imposed nutritional immunity.
Keywords: calprotectin; metallo-β-lactamases; metalloregulator; metallostasis; nutritional immunity.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

