Resveratrol alleviates intestinal mucosal barrier dysfunction in dextran sulfate sodium-induced colitis mice by enhancing autophagy
- PMID: 32952341
- PMCID: PMC7476174
- DOI: 10.3748/wjg.v26.i33.4945
Resveratrol alleviates intestinal mucosal barrier dysfunction in dextran sulfate sodium-induced colitis mice by enhancing autophagy
Abstract
Background: Intestinal mucosal barrier dysfunction plays an important role in the pathogenesis of ulcerative colitis (UC). Recent studies have revealed that impaired autophagy is associated with intestinal mucosal dysfunction in the mucosa of colitis mice. Resveratrol exerts anti-inflammatory functions by regulating autophagy.
Aim: To investigate the effect and mechanism of resveratrol on protecting the integrity of the intestinal mucosal barrier and anti-inflammation in dextran sulfate sodium (DSS)-induced ulcerative colitis mice.
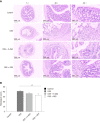
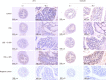
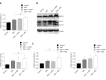
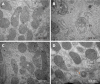
Methods: Male C57BL/6 mice were divided into four groups: negative control group, DSS model group, DSS + resveratrol group, and DSS + 5-aminosalicylic acid group. The severity of colitis was assessed by the disease activity index, serum inflammatory cytokines were detected by enzyme-linked immunosorbent assay. Colon tissues were stained with haematoxylin and eosin, and mucosal damage was evaluated by mean histological score. The expression of occludin and ZO-1 in colon tissue was evaluated using immunohistochemical analysis. In addition, the expression of autophagy-related genes was determined using reverse transcription-polymerase chain reaction and Western-blot, and morphology of autophagy was observed by transmission electron microscopy.
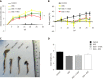
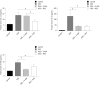
Results: The resveratrol treatment group showed a 1.72-fold decrease in disease activity index scores and 1.42, 3.81, and 1.65-fold decrease in the production of the inflammatory cytokine tumor necrosis factor-α, interleukin-6 and interleukin-1β, respectively, in DSS-induced colitis mice compared with DSS group (P < 0.05). The expressions of the tight junction proteins occludin and ZO-1 in DSS model group were decreased, and were increased in resveratrol-treated colitis group. Resveratrol also increased the levels of LC3B (by 1.39-fold compared with DSS group) and Beclin-1 (by 1.49-fold compared with DSS group) (P < 0.05), as well as the number of autophagosomes, which implies that the resveratrol may alleviate intestinal mucosal barrier dysfunction in DSS-induced UC mice by enhancing autophagy.
Conclusion: Resveratrol treatment decreased the expression of inflammatory factors, increased the expression of tight junction proteins and alleviated UC intestinal mucosal barrier dysfunction; this effect may be achieved by enhancing autophagy in intestinal epithelial cells.
Keywords: Autophagy; Intestinal inflammation; Intestinal mucosal barrier;Dextran sulfate sodium-induced colitis; Resveratrol; Ulcerative colitis.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: No potential conflicts of interest exist.
Figures
Similar articles
-
Effect of toll-like receptor 3 agonist poly I:C on intestinal mucosa and epithelial barrier function in mouse models of acute colitis.World J Gastroenterol. 2017 Feb 14;23(6):999-1009. doi: 10.3748/wjg.v23.i6.999. World J Gastroenterol. 2017. PMID: 28246473 Free PMC article.
-
Resveratrol ameliorates ulcerative colitis by upregulating Nrf2/HO‑1 pathway activity: Integrating animal experiments and network pharmacology.Mol Med Rep. 2024 May;29(5):77. doi: 10.3892/mmr.2024.13201. Epub 2024 Mar 15. Mol Med Rep. 2024. PMID: 38488031
-
Pulsatilla decoction improves DSS-induced colitis via modulation of fecal-bacteria-related short-chain fatty acids and intestinal barrier integrity.J Ethnopharmacol. 2023 Jan 10;300:115741. doi: 10.1016/j.jep.2022.115741. Epub 2022 Sep 24. J Ethnopharmacol. 2023. PMID: 36162543
-
Paeoniflorin promotes intestinal stem cell-mediated epithelial regeneration and repair via PI3K-AKT-mTOR signalling in ulcerative colitis.Int Immunopharmacol. 2023 Jun;119:110247. doi: 10.1016/j.intimp.2023.110247. Epub 2023 May 7. Int Immunopharmacol. 2023. PMID: 37159966 Review.
-
Anti-inflammatory effects of Inonotus obliquus in colitis induced by dextran sodium sulfate.J Biomed Biotechnol. 2010;2010:943516. doi: 10.1155/2010/943516. Epub 2010 Mar 10. J Biomed Biotechnol. 2010. PMID: 20300439 Free PMC article. Review.
Cited by
-
Harnessing the Anti-Inflammatory Properties of Polyphenols in the Treatment of Inflammatory Bowel Disease.Int J Biol Sci. 2024 Oct 14;20(14):5608-5672. doi: 10.7150/ijbs.98107. eCollection 2024. Int J Biol Sci. 2024. PMID: 39494333 Free PMC article. Review.
-
A potential therapeutic approach for ulcerative colitis: targeted regulation of mitochondrial dynamics and mitophagy through phytochemicals.Front Immunol. 2025 Jan 7;15:1506292. doi: 10.3389/fimmu.2024.1506292. eCollection 2024. Front Immunol. 2025. PMID: 39840057 Free PMC article. Review.
-
Dietary Grape Seed Meal Bioactive Compounds Alleviate Epithelial Dysfunctions and Attenuates Inflammation in Colon of DSS-Treated Piglets.Foods. 2021 Mar 4;10(3):530. doi: 10.3390/foods10030530. Foods. 2021. PMID: 33806347 Free PMC article.
-
Silencing ANGPT2 alleviates ulcerative colitis by regulating autophagy-mediated NLRP3 inflammasome inactivation via the mTOR signaling pathway.Braz J Med Biol Res. 2024 May 20;57:e13379. doi: 10.1590/1414-431X2024e13379. eCollection 2024. Braz J Med Biol Res. 2024. PMID: 38808888 Free PMC article.
-
Pingwei San Ameliorates Spleen Deficiency-Induced Diarrhea through Intestinal Barrier Protection and Gut Microbiota Modulation.Antioxidants (Basel). 2023 May 19;12(5):1122. doi: 10.3390/antiox12051122. Antioxidants (Basel). 2023. PMID: 37237988 Free PMC article.
References
-
- Sairenji T, Collins KL, Evans DV. An Update on Inflammatory Bowel Disease. Prim Care. 2017;44:673–692. - PubMed
-
- Yu YR, Rodriguez JR. Clinical presentation of Crohn's, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26:349–355. - PubMed
-
- Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. - PubMed
-
- Garcia-Planella E, Mañosa M, Van Domselaar M, Gordillo J, Zabana Y, Cabré E, López San Román A, Domènech E. Long-term outcome of ulcerative colitis in patients who achieve clinical remission with a first course of corticosteroids. Dig Liver Dis. 2012;44:206–210. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

