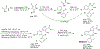Improved scale-up synthesis and purification of clinical asthma candidate MIDD0301
- PMID: 32952391
- PMCID: PMC7497793
- DOI: 10.1021/acs.oprd.0c00200
Improved scale-up synthesis and purification of clinical asthma candidate MIDD0301
Abstract
We report an improved and scalable synthesis of MIDD0301, a positive GABAA receptor modulator that is under development as oral and inhaled treatments for asthma. In contrast to other benzodiazepines in clinical use, MIDD0301 is a chiral compound that has limited brain absorption. The starting material to generate MIDD0301 is 2-amino-5-bromo-2'-fluorobenzophenone, which has a non-basic nitrogen due to electron withdrawing substituents in the ortho and para positions, reducing its reactivity towards activated carboxylic acids. Investigations of peptide coupling reagents on multigram scale resulted in moderate yields due to incomplete conversions. Secondly, basic conditions used for the formation of the seven-membered 1,4-diazepine ring resulted in racemization of the chiral center. We found that neutral conditions comparable to the pKa of the primary amine were sufficient to support the formation of the intramolecular imine but did not enable the simultaneous removal of the protecting group. Both difficulties were overcome with the application of the N-carboxyanhydride of D-alanine. Activated in the presence of acid, this compound reacted with non-basic 2-amino-5-bromo-2'-fluorobenzophenone and formed the 1,4-diazepine upon neutralization with triethylamine. Carefully designed workup procedures and divergent solubility of the synthetic intermediates in solvents and solvent combinations were utilized to eliminate the need for column chromatography. To improve compatibility with large scale reactors, temperature-controlled slow addition of reagents generated the imidazodiazepine at -20 °C. All intermediates were isolated with a purity of >97% and impurities were identified and quantified. After the final hydrolysis step, MIDD0301 was isolated in a 44% overall yield and purity of 98.9% after recrystallization. The enantiomeric excess was greater than 99.0%.
Keywords: GABAA receptor; amino acid N-carboxyanhydride; asthma; imidazodiazepine.
Conflict of interest statement
The authors declare the following competing financial interest(s): Drs. Stafford, Cook and Arnold are inventors of patent application WO2018035246A1, Gaba(A) receptor modulators and methods to control airway hyperresponsiveness and inflammation in asthma. Stafford and Arnold have equity interests in Pantherics Incorporated, which has certain intellectual property rights to these patents.
Figures
References
-
- Fryer RI, and Walser A Diazepine derivatives. Patent DE2540522, 1976.
-
- Walser A, Flynn T, and Fryer RI Quinazolines and 1,4-benzodiazepines. LXXXV. Syntheses of 3-substituted imidazo[1,5-a][1,4]benzodiazepines. J. Heterocycl. Chem 1978, 15, 577–583.
-
- Walser A, Fryer RI, Sternbach LH, and Archer MC Quinazolines and 1,4‐benzodiazepines. LXV some transformations of chlordiazepoxide. J. Heterocycl. Chem 1974, 11, 619–621.
-
- Ning RY, Fryer RI, Madan PB, and Sluboski BC Quinazolines and 1,4-Benzodiazepines. 74. Phosphorylation of Ambident Anions. Preparation of Some Di-4-morpholinylphosphinyloxy Imines via -Phosphorylation of Anions of Lactams. J. Org. Chem 1976, 41, 2720–2724.
-
- Buehler E, and Brown GB A general synthesis of N-hydroxyamino acids. J. Org. Chem 1967, 32, 265–267.
Grants and funding
LinkOut - more resources
Full Text Sources