Heavy iron isotope composition of iron meteorites explained by core crystallization
- PMID: 32952605
- PMCID: PMC7500481
- DOI: 10.1038/s41561-020-0617-y
Heavy iron isotope composition of iron meteorites explained by core crystallization
Abstract
Similar to Earth, many large planetesimals in the Solar System experienced planetary-scale processes such as accretion, melting, and differentiation. As their cores cooled and solidified, significant chemical fractionation occurred due to solid metal-liquid metal fractionation. Iron meteorites -- core remnants of these ancient planetesimals -- record a history of this process. Recent Fe isotope analyses of iron meteorites found δ57/54Fe to be heavier than chondritic by approximately 0.1 to 0.2 ‰ for most meteorites, indicating that a common parent body process was responsible. However, the mechanism for this fractionation remains poorly understood. Here we experimentally show that the Fe isotopic composition of iron meteorites can be explained solely by core crystallization. In our experiments of core crystallization at 1300 °C, we find that solid metal becomes enriched in δ57/54Fe by 0.13 ‰ relative to liquid metal. Fractional crystallization modelling of the IIIAB iron meteorite parent body shows that observed Ir, Au and Fe isotopic compositions can be simultaneously reproduced during core crystallization. The model implies the formation of complementary S-rich components of the iron meteorite parental cores that remain unsampled by meteorite records and may be the missing reservoir of isotopically-light Fe. The lack of sulfide meteorites and previous trace element modeling predicting significant unsampled volumes of iron meteorite parent cores support our findings.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures


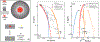

References
-
- Shahar A et al. Pressure-dependent isotopic composition of iron alloys. Science 352, 580–582 (2016). - PubMed
-
- Elardo SM & Shahar A Non-chondritic iron isotope ratios in planetary mantles as a result of core formation. Nat. Geosci 10, 317–321 (2017).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
