Treatment of anemia in cancer patients undergoing chemotherapy with intravenous ferric carboxymaltose without erythropoiesis-stimulating agents
- PMID: 32952616
- PMCID: PMC7485004
- DOI: 10.1177/1758835920953292
Treatment of anemia in cancer patients undergoing chemotherapy with intravenous ferric carboxymaltose without erythropoiesis-stimulating agents
Abstract
Background: Anemia is commonly encountered in cancer patients receiving active chemotherapy. Due to adverse events and presumed negative effects on disease-progression and survival, erythropoiesis-stimulating agents are not frequently used. In this study, we assess the efficacy and safety of intravenous ferric carboxymaltose (FCM) to treat cancer-induced anemia (CIA).
Patients and methods: We recruited adult cancer patients on active chemotherapy with a hemoglobin (Hb) level ⩽11.0 g/dL. Based on serum ferritin (sFr) and transferrin saturation (TSAT), patients were divided into 3 groups: group I (absolute iron deficiency, n = 26) with sFr < 30 ng/mL and TSAT < 20%; group II (functional iron deficiency, n = 24) with sFr 30-800 ng/mL and TSAT < 20%; and patients with TSAT ⩾ 20% were placed in group III as "others" (n = 34). All patients were treated with intravenous FCM. Serum hepcidin and C-reactive protein were used as biomarkers to predict response.
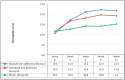
Results: A total of 84 patients with a median age (SD) of 53.8 (10.6) were recruited. Baseline median Hb level was 10.2 (range: 8.3-11.0) gm/dL. At week 12, there was a significant increment in Hb level for patients in groups I and II (median increment: 2.35 and 1.5 gm/dL, respectively), with limited response observed in group III, and most of the increment noted as early as week 3 (⩾1.0 g/dL). Responders tended to have lower levels of hepcidin. No clinically significant adverse events were reported; however, asymptomatic hypophosphatemia was observed in 39 (46.4%) patients.
Conclusions: Intravenous FCM is a safe and effective treatment option for the management of a subgroup of patients with CIA.The study was registered at ClinicalTrials.gov [Identifier: NCT04246021].
Keywords: anemia; cancer; ferric carboxymaltose; hepcidin.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Efficacy and safety of ferric carboxymaltose infusion in reducing anemia in patients receiving chemotherapy for nonmyeloid malignancies: A randomized, placebo-controlled study (IRON-CLAD).Am J Hematol. 2021 Dec 1;96(12):1639-1646. doi: 10.1002/ajh.26376. Epub 2021 Nov 19. Am J Hematol. 2021. PMID: 34653287 Free PMC article. Clinical Trial.
-
Efficacy of intravenous iron treatment for chemotherapy-induced anemia: A prospective Phase II pilot clinical trial in South Korea.PLoS Med. 2020 Jun 8;17(6):e1003091. doi: 10.1371/journal.pmed.1003091. eCollection 2020 Jun. PLoS Med. 2020. PMID: 32511251 Free PMC article. Clinical Trial.
-
Oxidative stress markers in predicting response to treatment with ferric carboxymaltose in nondialysis chronic kidney disease patients.Clin Nephrol. 2014 Jun;81(6):419-26. doi: 10.5414/CN108166. Clin Nephrol. 2014. PMID: 24691014
-
Functional iron deficiency anemia in patients with cancer.Blood Res. 2024 Aug 7;59(1):26. doi: 10.1007/s44313-024-00030-w. Blood Res. 2024. PMID: 39110268 Free PMC article. Review.
-
Ferric carboxymaltose: a review of its use in iron-deficiency anaemia.Drugs. 2009;69(6):739-56. doi: 10.2165/00003495-200969060-00007. Drugs. 2009. PMID: 19405553 Review.
Cited by
-
Seven-Year Single-Center Experience of the Efficacy and Safety of Ferric Carboxymaltose in Cancer Patients with Iron-Deficiency Anemia.Curr Oncol. 2023 Nov 2;30(11):9689-9700. doi: 10.3390/curroncol30110703. Curr Oncol. 2023. PMID: 37999123 Free PMC article.
-
Exercise Counteracts the Deleterious Effects of Cancer Cachexia.Cancers (Basel). 2022 May 19;14(10):2512. doi: 10.3390/cancers14102512. Cancers (Basel). 2022. PMID: 35626116 Free PMC article. Review.
-
Efficacy of ferric carboxymaltose or darbepoetin alfa for chemotherapy-induced anemia in patients with esophagogastric or pancreaticobiliary cancer: a retrospective comparative study.Ther Adv Med Oncol. 2024 Jul 31;16:17588359241265209. doi: 10.1177/17588359241265209. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39091605 Free PMC article.
-
The baseline hemoglobin level is a positive biomarker for immunotherapy response and can improve the predictability of tumor mutation burden for immunotherapy response in cancer.Front Pharmacol. 2024 Oct 2;15:1456833. doi: 10.3389/fphar.2024.1456833. eCollection 2024. Front Pharmacol. 2024. PMID: 39415833 Free PMC article.
-
Hypophosphataemia, fibroblast growth factor 23 and third-generation intravenous iron compounds: a narrative review.Drugs Context. 2021 Jan 19;10:2020-11-3. doi: 10.7573/dic.2020-11-3. eCollection 2021. Drugs Context. 2021. PMID: 33519940 Free PMC article. Review.
References
-
- Ludwig H, Van Belle S, Barrett-Lee P, et al. The European cancer anaemia survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004; 40: 2293–2306. - PubMed
-
- Grotto H. Anaemia of cancer: an overview of mechanisms involved in its pathogenesis. Med Oncol 2007; 25: 12–21. - PubMed
-
- Abdel-Razeq HN. Cancer-related anemia. Saudi Med J 2004; 25: 15–20. - PubMed
-
- Crawford J, Cella D, Cleeland C, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer 2002; 95: 888–895. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials