Composite ammonium glycyrrhizin has hepatoprotective effects in chicken hepatocytes with lipopolysaccharide/enrofloxacin-induced injury
- PMID: 32952642
- PMCID: PMC7485299
- DOI: 10.3892/etm.2020.9180
Composite ammonium glycyrrhizin has hepatoprotective effects in chicken hepatocytes with lipopolysaccharide/enrofloxacin-induced injury
Abstract
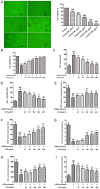
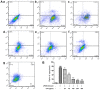
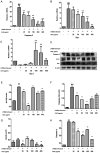
Composite ammonium glycyrrhizin (CAG) has anti-inflammatory activity. Lipopolysaccharide (LPS) and enrofloxacin (ENR) induce liver damage; however, the mechanism underlying LPS/ENR-induced hepatic injury remains to be elucidated. In the present study, the mechanism of LPS/ENR-induced liver injury was investigated in vitro and the protective effects of CAG were also evaluated. Primary chicken hepatocytes were isolated and a model of LPS/ENR-induced hepatocyte injury was established. mRNA and protein expression levels were evaluated by reverse transcription-quantitative polymerase chain reaction and western blot, respectively. LPS/ENR exposure significantly increased supernatant aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In the LPS/ENR-treated group, glutathione (GSH) and the antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activities were significantly increased. Flow cytometry results revealed that the apoptotic rate significantly increased in the LPS/ENR-treated group compared with the control, while treatment with CAG given 24 h prior to LPS/ENR caused a significant decrease in the apoptotic rate compared with the model group. Furthermore, CAG treatment reversed LPS/ENR-associated alterations in the mRNA and protein expression of Caspase-3, apoptosis regulator Bcl-2 (Bcl-2) and Bcl-2 associated X-protein. The mitochondrial membrane potential significantly decreased and the mitochondrial microstructure was notably altered following exposure to LPS/ENR compared with the control. In conclusion, these results suggested that LPS/ENR-treated hepatocytes were damaged via apoptotic signaling pathways and CAG prevented LPS/ENR-induced hepatocyte injury.
Keywords: composite ammonium glycyrrhizim; drug protection; lipopolysaccharide/enrofloxacin; liver injury.
Copyright: © Guo et al.
Figures

Similar articles
-
Compound Ammonium Glycyrrhizin Protects Hepatocytes from Injury Induced by Lipopolysaccharide/Florfenicol through a Mitochondrial Pathway.Molecules. 2018 Sep 17;23(9):2378. doi: 10.3390/molecules23092378. Molecules. 2018. PMID: 30227687 Free PMC article.
-
Ammonium glycyrrhizin counteracts liver injury caused by lipopolysaccharide/amoxicillin-clavulanate potassium.Oncotarget. 2017 May 30;8(57):96837-96851. doi: 10.18632/oncotarget.18291. eCollection 2017 Nov 14. Oncotarget. 2017. PMID: 29228575 Free PMC article.
-
Protective effects of compound ammonium glycyrrhizin, L‑arginine, silymarin and glucurolactone against liver damage induced by ochratoxin A in primary chicken hepatocytes.Mol Med Rep. 2018 Sep;18(3):2551-2560. doi: 10.3892/mmr.2018.9285. Epub 2018 Jul 16. Mol Med Rep. 2018. PMID: 30015927 Free PMC article.
-
Compound ammonium glycyrrhizin protects hepatocytes from injury induced by lipopolysaccharide/florfenicol through oxidative stress and a MAPK pathway.Comp Biochem Physiol C Toxicol Pharmacol. 2019 Nov;225:108585. doi: 10.1016/j.cbpc.2019.108585. Epub 2019 Aug 6. Comp Biochem Physiol C Toxicol Pharmacol. 2019. PMID: 31398390
-
Mechanism for hepato-protective action of Liangxue Huayu Recipe (LHR): blockade of mitochondrial cytochrome c release and caspase activation.J Ethnopharmacol. 2013 Jul 30;148(3):851-60. doi: 10.1016/j.jep.2013.05.024. Epub 2013 May 24. J Ethnopharmacol. 2013. PMID: 23711831
Cited by
-
The Research Status, Potential Hazards and Toxicological Mechanisms of Fluoroquinolone Antibiotics in the Environment.Antibiotics (Basel). 2023 Jun 15;12(6):1058. doi: 10.3390/antibiotics12061058. Antibiotics (Basel). 2023. PMID: 37370377 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
