A novel definition of microvessel density in renal cell carcinoma: Angiogenesis plus vasculogenic mimicry
- PMID: 32952661
- PMCID: PMC7479517
- DOI: 10.3892/ol.2020.12054
A novel definition of microvessel density in renal cell carcinoma: Angiogenesis plus vasculogenic mimicry
Abstract
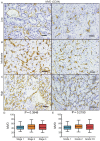
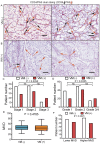
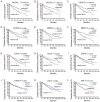
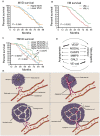
The present study proposed the novel concept of total microvessel density (TMVD), which is the combination of the MVD and the vasculogenic mimicry (VM) status, and evaluated its clinical significance in patients with renal cell carcinoma (RCC). For that purpose, tumor samples from 183 patients with primary RCC were examined by CD34 single or periodic acid Schiff (PAS)/CD34 dual histology staining. MVD and VM were determined according to previous literature. Clinical information (tumor stage and grade, and duration of survival) was retrieved and analyzed. Survival information and VM-associated gene expression data of patients with RCC were also retrieved from The Cancer Genome Atlas (TCGA) database and the clinical significance of each individual gene was analyzed. The results indicated that MVD exhibited obvious differences among patients with RCC; however, it was not correlated with the stage/grade or length of survival in patients with RCC. In total, 81 patients (44.3%) were CD34(-)/PAS(+) and defined as VM(+), and they had a significantly shorter survival compared with that of VM(-) patients (P=0.0002). VM was not associated with MVD. TMVD was able to distinguish between patients with high and low MVD in terms of survival, thus TMVD was better compared with MVD alone at distinguishing between patients with different survival prognoses. TCGA data analysis revealed that among the VM-associated genes, nodal growth differentiation factor, caspase-3, matrix metalloproteinase-9 and galectin-3 had a statistically significant impact on the overall/disease-free survival of patients with RCC. In conclusion, the TMVD concept may be more appropriate and sensitive compared with the MVD or VM alone in predicting tumor aggressiveness and patient survival, particularly in RCC, which is a highly vascularized, VM-rich neoplasm, and certain VM formation-associated genes are negatively associated with the survival of patients with RCC.
Keywords: TCGA database; immunohistochemical staining; microvessel density; renal cell carcinoma; vasculogenic mimicry.
Copyright: © Wu et al.
Figures
Similar articles
-
N-myc downstream-regulated gene 1 can promote vasculogenic mimicry and angiogenesis in urothelial carcinoma.Virchows Arch. 2024 May;484(5):827-836. doi: 10.1007/s00428-024-03793-w. Epub 2024 Apr 2. Virchows Arch. 2024. PMID: 38561462 Free PMC article.
-
Clinical significances and prognostic value of cancer stem-like cells markers and vasculogenic mimicry in renal cell carcinoma.J Surg Oncol. 2013 Nov;108(6):414-9. doi: 10.1002/jso.23402. Epub 2013 Aug 29. J Surg Oncol. 2013. PMID: 23996537
-
Matrix metalloproteinase-9 is required for vasculogenic mimicry by clear cell renal carcinoma cells.Urol Oncol. 2015 Apr;33(4):168.e9-16. doi: 10.1016/j.urolonc.2014.12.007. Epub 2015 Jan 21. Urol Oncol. 2015. PMID: 25618297
-
An Overview of Vasculogenic Mimicry in Breast Cancer.Front Oncol. 2020 Feb 27;10:220. doi: 10.3389/fonc.2020.00220. eCollection 2020. Front Oncol. 2020. PMID: 32175277 Free PMC article. Review.
-
Therapeutic potential of vasculogenic mimicry in urological tumors.Front Oncol. 2023 Sep 21;13:1202656. doi: 10.3389/fonc.2023.1202656. eCollection 2023. Front Oncol. 2023. PMID: 37810976 Free PMC article. Review.
Cited by
-
Exploring vasculogenesis in the normal human kidney and clear cell renal cell carcinoma: insights from development to tumor progression and biomarkers for therapy response.Front Oncol. 2024 Apr 30;14:1375190. doi: 10.3389/fonc.2024.1375190. eCollection 2024. Front Oncol. 2024. PMID: 38746686 Free PMC article. Review.
-
Identification of VEGFs-related gene signature for predicting microangiogenesis and hepatocellular carcinoma prognosis.Aging (Albany NY). 2024 Jun 13;16(12):10321-10347. doi: 10.18632/aging.205931. Epub 2024 Jun 13. Aging (Albany NY). 2024. PMID: 38874512 Free PMC article.
-
Prognostic and predictive significance of VEGF, CD31, and Ang-1 in patients with metastatic clear cell renal cell carcinoma treated with first-line sunitinib.Biomol Biomed. 2023 Feb 1;23(1):161-169. doi: 10.17305/bjbms.2022.7675. Biomol Biomed. 2023. PMID: 35674770 Free PMC article.
-
N-myc downstream-regulated gene 1 can promote vasculogenic mimicry and angiogenesis in urothelial carcinoma.Virchows Arch. 2024 May;484(5):827-836. doi: 10.1007/s00428-024-03793-w. Epub 2024 Apr 2. Virchows Arch. 2024. PMID: 38561462 Free PMC article.
-
EGR1 Regulation of Vasculogenic Mimicry in the MDA-MB-231 Triple-Negative Breast Cancer Cell Line through the Upregulation of KLF4 Expression.Int J Mol Sci. 2023 Sep 21;24(18):14375. doi: 10.3390/ijms241814375. Int J Mol Sci. 2023. PMID: 37762678 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
