Photosensitized direct C-H fluorination and trifluoromethylation in organic synthesis
- PMID: 32952732
- PMCID: PMC7476599
- DOI: 10.3762/bjoc.16.183
Photosensitized direct C-H fluorination and trifluoromethylation in organic synthesis
Abstract
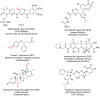
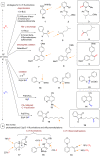
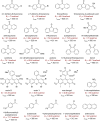
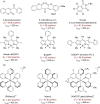
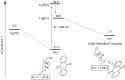
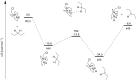
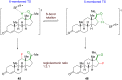
The importance of fluorinated products in pharmaceutical and medicinal chemistry has necessitated the development of synthetic fluorination methods, of which direct C-H fluorination is among the most powerful. Despite the challenges and limitations associated with the direct fluorination of unactivated C-H bonds, appreciable advancements in manipulating the selectivity and reactivity have been made, especially via transition metal catalysis and photochemistry. Where transition metal catalysis provides one strategy for C-H bond activation, transition-metal-free photochemical C-H fluorination can provide a complementary selectivity via a radical mechanism that proceeds under milder conditions than thermal radical activation methods. One exciting development in C-F bond formation is the use of small-molecule photosensitizers, allowing the reactions i) to proceed under mild conditions, ii) to be user-friendly, iii) to be cost-effective and iv) to be more amenable to scalability than typical photoredox-catalyzed methods. In this review, we highlight photosensitized C-H fluorination as a recent strategy for the direct and remote activation of C-H (especially C(sp3)-H) bonds. To guide the readers, we present the developing mechanistic understandings of these reactions and exemplify concepts to assist the future planning of reactions.
Keywords: C–H activation; energy transfer; fluorination; photocatalysis; photosensitization; visible light.
Copyright © 2020, Yakubov and Barham; licensee Beilstein-Institut.
Figures






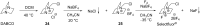
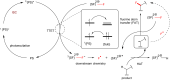
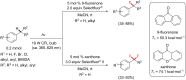
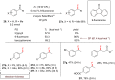


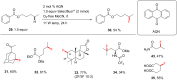

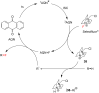
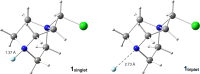




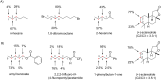



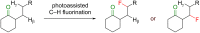

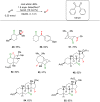
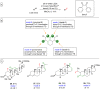



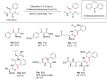

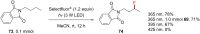

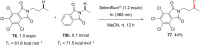
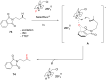

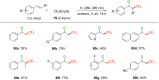



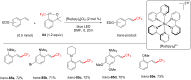
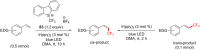
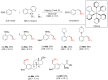

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
