Switchable foldamer ion channels with antibacterial activity
- PMID: 32953034
- PMCID: PMC7481839
- DOI: 10.1039/d0sc02393k
Switchable foldamer ion channels with antibacterial activity
Abstract
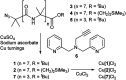
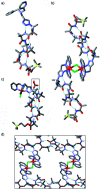
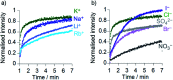
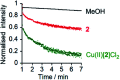
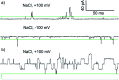
Synthetic ion channels may have applications in treating channelopathies and as new classes of antibiotics, particularly if ion flow through the channels can be controlled. Here we describe triazole-capped octameric α-aminoisobutyric acid (Aib) foldamers that "switch on" ion channel activity in phospholipid bilayers upon copper(ii) chloride addition; activity is "switched off" upon copper(ii) extraction. X-ray crystallography showed that CuCl2 complexation gave chloro-bridged foldamer dimers, with hydrogen bonds between dimers producing channels within the crystal structure. These interactions suggest a pathway for foldamer self-assembly into membrane ion channels. The copper(ii)-foldamer complexes showed antibacterial activity against B. megaterium strain DSM319 that was similar to the peptaibol antibiotic alamethicin, but with 90% lower hemolytic activity.
This journal is © The Royal Society of Chemistry 2020.
Figures



References
-
- Ptáček L. J. Neuromuscular Disord. 1997;7:250–255. - PubMed
-
- Matile S., Sakai N. and Hennig A., Transport Experiments in Membranes, in Supramolecular Chemistry: From Molecules to Nanomaterials, John Wiley & Sons, Ltd, 2012.
-
- Davis A. P., Sheppard D. N., Smith B. D. Chem. Soc. Rev. 2007;36:348–357. - PMC - PubMed
- Busschaert N., Park S.-H., Baek K.-H., Choi Y. P., Park J., Howe E. N. W., Hiscock J. R., Karagiannidis L. E., Marques I., Félix V., Namkung W., Sessler J. L., Gale P. A., Shin I. Nat. Chem. 2017;9:667–675. - PMC - PubMed
- Gale P. A., Davis J. T., Quesada R. Chem. Soc. Rev. 2017;46:2497–2519. - PubMed
- Li H., Valkenier H., Judd L. W., Brotherhood P. R., Hussain S., Cooper J. A., Jurček O., Sparkes H. A., Sheppard D. N., Davis A. P. Nat. Chem. 2016;8:24–32. - PubMed
- Alfonso I., Quesada R. Chem. Sci. 2013;4:3009–3019.
- Behera H., Madhavan N. J. Am. Chem. Soc. 2017;139:12919–12922. - PubMed
LinkOut - more resources
Full Text Sources

