Seasonal morphotypes of Drosophila suzukii differ in key life-history traits during and after a prolonged period of cold exposure
- PMID: 32953048
- PMCID: PMC7487234
- DOI: 10.1002/ece3.6517
Seasonal morphotypes of Drosophila suzukii differ in key life-history traits during and after a prolonged period of cold exposure
Abstract
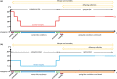
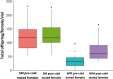
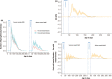
Seasonal polyphenism in Drosophila suzukii manifests itself in two discrete adult morphotypes, the "winter morph" (WM) and the "summer morph" (SM). These morphotypes are known to differ in thermal stress tolerance, and they co-occur during parts of the year. In this study, we aimed to estimate morph-specific survival and fecundity in laboratory settings simulating field conditions. We specifically analyzed how WM and SM D. suzukii differed in mortality and reproduction during and after a period of cold exposure resembling winter and spring conditions in temperate climates. The median lifespan of D. suzukii varied around 5 months for the WM flies and around 7 months for the SM flies. WM flies showed higher survival during the cold-exposure period compared with SM flies, and especially SM males suffered high mortality under these conditions. In contrast, SM flies had lower mortality rates than WM flies under spring-like conditions. Intriguingly, reproductive status (virgin or mated) did not impact the fly survival, either during the cold exposure or during spring-like conditions. Even though the reproductive potential of WM flies was greatly reduced compared with SM flies, both WM and SM females that had mated before the cold exposure were able to continuously produce viable offspring for 5 months under spring-like conditions. Finally, the fertility of the overwintered WM males was almost zero, while the surviving SM males did not suffer reduced fertility. Combined with other studies on D. suzukii monitoring and overwintering behavior, these results suggest that overwintered flies of both morphotypes could live long enough to infest the first commercial crops of the season. The high mortality of SM males and the low fertility of WM males after prolonged cold exposure also highlight the necessity for females to store sperm over winter to be able to start reproducing early in the following spring.
Keywords: Drosophila suzukii; fertility; life history; overwintering; reproduction; seasonal polyphenism; spotted‐wing drosophila; survival.
© 2020 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Adaptation of an Invasive Pest to Novel Environments: Life History Traits of Drosophila suzukii in Coastal and Mainland Areas of Greece during Overwintering.Biology (Basel). 2021 Jul 29;10(8):727. doi: 10.3390/biology10080727. Biology (Basel). 2021. PMID: 34439959 Free PMC article.
-
Survival and Fecundity Parameters of Two Drosophila suzukii (Diptera: Drosophilidae) Morphs on Variable Diet Under Suboptimal Temperatures.J Insect Sci. 2018 Nov 1;18(6):8. doi: 10.1093/jisesa/iey113. J Insect Sci. 2018. PMID: 30445636 Free PMC article.
-
Interactions among morphotype, nutrition, and temperature impact fitness of an invasive fly.Ecol Evol. 2019 Feb 3;9(5):2615-2628. doi: 10.1002/ece3.4928. eCollection 2019 Mar. Ecol Evol. 2019. PMID: 31061698 Free PMC article.
-
Winter Is (Not) Coming: Is Climate Change Helping Drosophila suzukii Overwintering?Biology (Basel). 2023 Jun 25;12(7):907. doi: 10.3390/biology12070907. Biology (Basel). 2023. PMID: 37508339 Free PMC article. Review.
-
Advances in the Chemical Ecology of the Spotted Wing Drosophila (Drosophila suzukii) and its Applications.J Chem Ecol. 2018 Oct;44(10):922-939. doi: 10.1007/s10886-018-1000-y. Epub 2018 Jul 27. J Chem Ecol. 2018. PMID: 30054769 Review.
Cited by
-
Behavior Individuality: A Focus on Drosophila melanogaster.Front Physiol. 2021 Nov 30;12:719038. doi: 10.3389/fphys.2021.719038. eCollection 2021. Front Physiol. 2021. PMID: 34916952 Free PMC article. Review.
-
Molecular and behavioral studies reveal differences in olfaction between winter and summer morphs of Drosophila suzukii.PeerJ. 2022 Sep 16;10:e13825. doi: 10.7717/peerj.13825. eCollection 2022. PeerJ. 2022. PMID: 36132222 Free PMC article.
-
Seasonal changes in photoperiod and temperature lead to changes in cuticular hydrocarbon profiles and affect mating success in Drosophila suzukii.Sci Rep. 2023 Apr 6;13(1):5649. doi: 10.1038/s41598-023-32652-y. Sci Rep. 2023. PMID: 37024537 Free PMC article.
-
The Critical Impact of Sub- and Supraoptimal Temperatures on Male Fertility Potential of an Invasive Fruit Fly.Ecol Evol. 2025 Jun 4;15(6):e71515. doi: 10.1002/ece3.71515. eCollection 2025 Jun. Ecol Evol. 2025. PMID: 40475867 Free PMC article.
-
The contrasting role of climate variation on the population dynamics of a native and an invasive insect pest.PLoS One. 2023 Apr 28;18(4):e0284600. doi: 10.1371/journal.pone.0284600. eCollection 2023. PLoS One. 2023. PMID: 37115782 Free PMC article.
References
-
- Arnó, J. , Solà, M. , Riudavets, J. , & Gabarra, R. (2016). Population dynamics, non‐crop hosts, and fruit susceptibility of Drosophila suzukii in Northeast Spain. Journal of Pest Science, 89(3), 713–723. 10.1007/s10340-016-0774-3 - DOI
-
- Asplen, M. K. , Anfora, G. , Biondi, A. , Choi, D. S. , Chu, D. , Daane, K. M. , … Desneux, N. (2015). Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. Journal of Pest Science, 88(3), 469–494. 10.1007/s10340-015-0681-z - DOI
-
- Bender, A. , Groll, A. , & Scheipl, F. (2018). A generalized additive model approach to time‐to‐event analysis. Statistical Modelling, 18, 299–321. 10.1177/1471082X17748083 - DOI
-
- Boulétreau‐Merle, J. , & Fouillet, P. (2002). How to overwinter and be a founder: Egg‐retention phenotypes and mating status in Drosophila melanogaster . Evolutionary Ecology, 16(4), 309–332. 10.1023/A:1020216230976 - DOI
-
- Briem, F. , Eben, A. , Gross, J. , & Vogt, H. (2016). An invader supported by a parasite: Mistletoe berries as a host for food and reproduction of spotted wing Drosophila in early spring. Journal of Pest Science, 89(3), 749–759. 10.1007/s10340-016-0739-6 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

