Long-Term Efficacy of Subcutaneous C1 Inhibitor in Pediatric Patients with Hereditary Angioedema
- PMID: 32953229
- PMCID: PMC7499895
- DOI: 10.1089/ped.2020.1143
Long-Term Efficacy of Subcutaneous C1 Inhibitor in Pediatric Patients with Hereditary Angioedema
Abstract
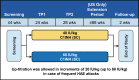
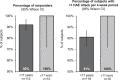
Background: Hereditary angioedema (HAE) due to C1 inhibitor (C1INH) deficiency is characterized by recurrent attacks of edema of the skin and mucosal tissues. Symptoms usually present during childhood (mean age at first attack, 10 years). Earlier symptom onset may predict a more severe disease course. Subcutaneous (SC) C1INH is indicated for routine prophylaxis to prevent HAE attacks in adolescents and adults. We analyzed the long-term efficacy of C1INH (SC) in subjects ≤17 years old treated in an open-label extension (OLE) of the pivotal phase III Clinical Study for Optimal Management of Preventing Angioedema with Low-Volume Subcutaneous C1 Inhibitor Replacement Therapy (COMPACT) trial. Methods: Eligible subjects (age ≥6 years, with ≥4 attacks over 2 consecutive months before entry into the OLE or placebo-controlled COMPACT trial) were treated with C1INH (SC) 40 or 60 IU/kg twice weekly for 52-140 weeks. Subgroup analyses by age (≤17 vs. >17 years) were performed for key efficacy endpoints. Results: Ten subjects were ≤17 years old [mean (range) age, 13.3 (8-16) years, 3 subjects <12 years old; exposure range, 51-133 weeks]. All 10 pediatric subjects experienced ≥50% reduction (mean, 93%) in number of attacks versus the prestudy period, with a 97% reduction in the median number of attacks/month (0.11). All subjects had <1 attack/4-week period and 4 had <1 attack/year (1 subject was attack free). No subject discontinued treatment due to a treatment-related adverse event. Conclusions: Data from pediatric subjects treated with C1INH (SC) for up to 2.55 years and adult subjects revealed similar efficacy. C1INH (SC) is effective and well tolerated as long-term prophylaxis in children, adolescents, and adults with HAE.
Keywords: C1 inhibitor; COMPACT; Haegarda; children; hereditary angioedema; long term; pediatric; prophylaxis; quality of life; safety.
© Donald Levy et al., 2020; Published by Mary Ann Liebert, Inc.
Conflict of interest statement
Donald S. Levy, is a consultant with Takeda, Biocryst, CSL Behring, Pharming; is a speaker for Takeda and CSL Behring; has received clinical research grants from CSL Behring. Teresa Caballero has received grant research, grant support and/or speaker/consultancy fees from BioCryst, CSL Behring, Merck, Novartis, Octapharma, Pharming, and Shire (a Takeda company); has received funding to attend conferences/educational events from CSL Behring, Novartis and Shire; is/has been a clinical trial/registry investigator for Biocryst, CSL Behring, Novartis, Pharming and Shire; and is a researcher from the IdiPAZ program for promoting research activities. Iftikhar Hussain is a speaker for CSL Behring, Grifols, and Optionse; is/has been on the advisory board for Blueprint Medicine and Pfizer; is Principal Investigator for Adare, Sanofi, Cara Therapeutics, Dermira, Lilly, Pfizer, Abbvie, CSL Behring, Astra-Zeneca, and Genentech, ALK. Avner Reshef has received research funding, travel grants, and/or speaker honorarium-consultation fee from CSL-Behring, Pharming, BioCryst, Takeda-Shire, Teva, Stallergens. John Anderson has potential conflicts of interest with CSL Behring, Takeda, BioCryst. James Baker has no conflicts of interest. Lawrence B. Schwartz is/has been HAE clinical trial principal investigator for CSL Behring and Takeda; is/has been on the advisory board for CSL Behring LLC, Takeda Pharmaceuticals, Pharming Healthcare, and BioCryst Pharmaceuticals. Marco Cicardi has potential conflicts of interest with CSL Behring, Shire, and Pharming. Subhransu Prusty, Henrike Feuersenger, and Ingo Pragst are/have been CSL Behring employees. Michael Manning has received grant support from CSL Behring, Takeda/Shire, BioCryst, and KalVista; is a speaker for CSL Behring, Takeda/Shire, and Pharming; is a consultant with and on the advisory boards for CSL Behring, Takeda/Shire, BioCryst, and Pharming.
Figures
Similar articles
-
Preventive Treatment of Hereditary Angioedema: A Review of Phase III Clinical Trial Data for Subcutaneous C1 Inhibitor and Relevance for Patient Management.Clin Ther. 2021 Dec;43(12):2154-2166.e1. doi: 10.1016/j.clinthera.2021.10.008. Epub 2021 Dec 5. Clin Ther. 2021. PMID: 34879971 Review.
-
Long-Term Outcomes with Subcutaneous C1-Inhibitor Replacement Therapy for Prevention of Hereditary Angioedema Attacks.J Allergy Clin Immunol Pract. 2019 Jul-Aug;7(6):1793-1802.e2. doi: 10.1016/j.jaip.2019.01.054. Epub 2019 Feb 15. J Allergy Clin Immunol Pract. 2019. PMID: 30772477 Clinical Trial.
-
Prophylactic therapy with subcutaneous C1-inhibitor is associated with sustained symptom control in patients with hereditary angioedema.Allergy Asthma Proc. 2022 May 1;43(3):202-208. doi: 10.2500/aap.2022.43.220016. Allergy Asthma Proc. 2022. PMID: 35524357 Clinical Trial.
-
Long-term efficacy and safety of subcutaneous C1-inhibitor in women with hereditary angioedema: subgroup analysis from an open-label extension of a phase 3 trial.Allergy Asthma Clin Immunol. 2020 Feb 4;16:8. doi: 10.1186/s13223-020-0409-3. eCollection 2020. Allergy Asthma Clin Immunol. 2020. PMID: 32042283 Free PMC article.
-
Update on the Use of C1-Esterase Inhibitor Replacement Therapy in the Acute and Prophylactic Treatment of Hereditary Angioedema.Clin Rev Allergy Immunol. 2019 Apr;56(2):207-218. doi: 10.1007/s12016-018-8684-1. Clin Rev Allergy Immunol. 2019. PMID: 29909591 Review.
Cited by
-
Indirect treatment comparison of lanadelumab and a C1-esterase inhibitor in pediatric patients with hereditary angioedema.J Comp Eff Res. 2025 Feb;14(2):e240110. doi: 10.57264/cer-2024-0110. Epub 2025 Jan 21. J Comp Eff Res. 2025. PMID: 39836016 Free PMC article.
-
Network Meta-Analysis of Pharmacological Therapies for Long-Term Prophylactic Treatment of Patients with Hereditary Angioedema.Drugs R D. 2025 Jun;25(2):161-178. doi: 10.1007/s40268-025-00511-y. Epub 2025 May 28. Drugs R D. 2025. PMID: 40434599 Free PMC article.
-
Interventions for the long-term prevention of hereditary angioedema attacks.Cochrane Database Syst Rev. 2022 Nov 3;11(11):CD013403. doi: 10.1002/14651858.CD013403.pub2. Cochrane Database Syst Rev. 2022. PMID: 36326435 Free PMC article.
-
Hereditary Angioedema Attacks in Patients Receiving Long-Term Prophylaxis: A Systematic Review.Clin Rev Allergy Immunol. 2024 Dec;67(1-3):83-95. doi: 10.1007/s12016-024-09006-1. Epub 2024 Nov 7. Clin Rev Allergy Immunol. 2024. PMID: 39508959 Free PMC article.
-
Hereditary angioedema in children: Review and practical perspective for clinical management.Pediatr Allergy Immunol. 2024 Dec;35(12):e14268. doi: 10.1111/pai.14268. Pediatr Allergy Immunol. 2024. PMID: 39655944 Free PMC article. Review.
References
-
- Zuraw BL. Hereditary angioedema. N Engl J Med 2008; 359:1027–1036 - PubMed
-
- Bork K, Meng G, Staubach P, et al. . Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med 2006; 119:267–274 - PubMed
-
- Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1INH deficiency. J Allergy Clin Immunol 2012; 130:692–697 - PubMed
-
- Bork K, Staubach P, Eckardt AJ, et al. . Symptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiency. Am J Gastroenterol 2006; 101:619–627 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
