Dynamics of Contrast Decrement and Increment Responses in Human Visual Cortex
- PMID: 32953246
- PMCID: PMC7476656
- DOI: 10.1167/tvst.9.10.6
Dynamics of Contrast Decrement and Increment Responses in Human Visual Cortex
Abstract
Purpose: The goal of the present experiments was to determine whether electrophysiologic response properties of the ON and OFF visual pathways observed in animal experimental models can be observed in humans.
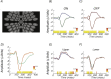
Methods: Steady-state visual evoked potentials (SSVEPs) were recorded in response to equivalent magnitude contrast increments and decrements presented within a probe-on-pedestal Westheimer sensitization paradigm. The probes were modulated with sawtooth temporal waveforms at a temporal frequency of 3 or 2.73 Hz. SSVEP response waveforms and response spectra for incremental and decremental stimuli were analyzed as a function of stimulus size and visual field location in 67 healthy adult participants.
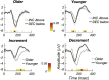
Results: SSVEPs recorded at the scalp differ between contrast decrements and increments of equal Weber contrast: SSVEP responses were larger in amplitude and shorter in latency for contrast decrements than for contrast increments. Both increment and decrement responses were larger for displays that were scaled for cortical magnification.
Conclusions: In a fashion that parallels results from the early visual system of cats and monkeys, two key properties of ON versus OFF pathways found in single-unit recordings are recapitulated at the population level of activity that can be observed with scalp electrodes, allowing differential assessment of ON and OFF pathway activity in human.
Translational relevance: As data from preclinical models of visual pathway dysfunction point to differential damage to subtypes of retinal ganglion cells, this approach may be useful in future work on disease detection and treatment monitoring.
Keywords: OFF pathway; ON pathway; latency; luminance contrast; visual evoked potentials.
Copyright 2020 The Authors.
Conflict of interest statement
Disclosure: A.M. Norcia, None; A. Yakovleva, None; B. Hung, None; J.L. Goldberg, None
Figures


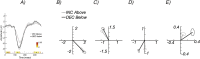




Similar articles
-
Visual Field Asymmetries in Responses to ON and OFF Pathway Biasing Stimuli.Vis Neurosci. 2024 Dec 19;41:E007. doi: 10.1017/S095252382400004X. Vis Neurosci. 2024. PMID: 39698978 Free PMC article.
-
Preferential Loss of Contrast Decrement Responses in Human Glaucoma.Invest Ophthalmol Vis Sci. 2022 Oct 3;63(11):16. doi: 10.1167/iovs.63.11.16. Invest Ophthalmol Vis Sci. 2022. PMID: 36264656 Free PMC article.
-
Steady-state visual evoked potentials: distributed local sources and wave-like dynamics are sensitive to flicker frequency.Brain Topogr. 2006 Spring;18(3):167-87. doi: 10.1007/s10548-006-0267-4. Epub 2006 Mar 1. Brain Topogr. 2006. PMID: 16544207 Free PMC article.
-
Steady-state visually evoked potentials: focus on essential paradigms and future perspectives.Prog Neurobiol. 2010 Apr;90(4):418-38. doi: 10.1016/j.pneurobio.2009.11.005. Epub 2009 Dec 4. Prog Neurobiol. 2010. PMID: 19963032 Review.
-
Spatial frequency analysis in the visual system.Annu Rev Neurosci. 1985;8:547-83. doi: 10.1146/annurev.ne.08.030185.002555. Annu Rev Neurosci. 1985. PMID: 3920946 Review. No abstract available.
Cited by
-
Visual Field Asymmetries in Responses to ON and OFF Pathway Biasing Stimuli.Vis Neurosci. 2024 Dec 19;41:E007. doi: 10.1017/S095252382400004X. Vis Neurosci. 2024. PMID: 39698978 Free PMC article.
-
Differences in visual stimulation between reading and walking and implications for myopia development.J Vis. 2023 Apr 3;23(4):3. doi: 10.1167/jov.23.4.3. J Vis. 2023. PMID: 37014657 Free PMC article.
-
Lexical and sublexical cortical tuning for print revealed by Steady-State Visual Evoked Potentials (SSVEPs) in early readers.Dev Sci. 2023 Jul;26(4):e13352. doi: 10.1111/desc.13352. Epub 2022 Dec 8. Dev Sci. 2023. PMID: 36413170 Free PMC article.
-
Luminance Contrast Shifts Dominance Balance between ON and OFF Pathways in Human Vision.J Neurosci. 2023 Feb 8;43(6):993-1007. doi: 10.1523/JNEUROSCI.1672-22.2022. Epub 2022 Dec 19. J Neurosci. 2023. PMID: 36535768 Free PMC article.
-
OPTICAL BLUR AFFECTS DIFFERENTLY ON AND OFF VISUAL PATHWAYS.bioRxiv [Preprint]. 2024 Oct 21:2024.10.17.618707. doi: 10.1101/2024.10.17.618707. bioRxiv. 2024. Update in: iScience. 2025 Apr 22;28(6):112500. doi: 10.1016/j.isci.2025.112500. PMID: 39484435 Free PMC article. Updated. Preprint.
References
-
- Dowling JE, Werblin FS. Synaptic organization of the vertebrate retina. Vision Res. 1971;(suppl 3): 1–15. - PubMed
-
- Famiglietti EV Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976; 194: 193–195. - PubMed
-
- Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004; 5: 747–757. - PubMed
-
- LeVay S, McConnell SK. ON and OFF layers in the lateral geniculate nucleus of the mink. Nature. 1982; 300: 350–351. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

