Neutrophil-to-lymphocyte ratio in combination with PD-L1 or lactate dehydrogenase as biomarkers for high PD-L1 non-small cell lung cancer treated with first-line pembrolizumab
- PMID: 32953525
- PMCID: PMC7481583
- DOI: 10.21037/tlcr-19-583
Neutrophil-to-lymphocyte ratio in combination with PD-L1 or lactate dehydrogenase as biomarkers for high PD-L1 non-small cell lung cancer treated with first-line pembrolizumab
Abstract
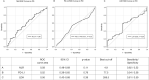
The identification of prognostic and predictive biomarkers for high-programmed cell death-ligand 1 (PD-L1) advanced non-small cell lung cancer (aNSCLC) treated with first-line pembrolizumab could support the decision-making about possible combination therapies. To explore the baseline neutrophil-to-lymphocyte ratio (NLR) with the possible addition of PD-L1 tumour proportion score (TPS) level or lactate dehydrogenase (LDH) as possible prognostic biomarkers by a multicenter retrospective exploratory analysis aiming at identifying favourable-risk patients. Baseline NLR was available for all 132 high PD-L1 aNSCLC patients, PD-L1 level and LDH for 81 (61%) and 85 (64%) patients, respectively. NLR, PD-L1 and LDH cut-offs by receiver operating characteristic (ROC) curves were 4.9, 77.5% and 268.5, respectively. Seventy-one patients (54%) had NLR <5; 25 out of 81 NLR <5 (31%) had PD-L1 >80%, 26 out of 85 (31%) NLR <5 and normal LDH (nLDH). Median follow-up was 16.3 months. As compared to NLR >5, significantly better 2-year overall survival (OS) and progression-free survival (PFS) were observed with NLR <5 [62% vs. 41%, P=0.005, hazard ratio (HR) 0.45, and median of 12.0 vs. 5.7 months, P=0.01, HR 0.56, respectively], NLR <5 + PD-L1 >80% (81%, P=0.006, HR 0.20 and median of 14.7, P=0.03, HR 0.44, respectively), and NLR <5 + nLDH (74%, P=0.009, HR 0.25 and median of 14.7, P=0.02, HR 0.40, respectively). NLR <5 and NLR <5 + nLDH significantly associated with PD (P=0.008 and P=0.025, respectively) but not response rate (RR) (P=0.09 and P=0.07, respectively); NLR <5 + PD-L1 >80% both RR (P=0.03) and PD (P=0.02). NLR <5 ± PD-L1 >80% or nLDH could represent easy-to-assess tools to identify high PD-L1 aNSCLC patients with favourable outcome following first-line pembrolizumab monotherapy.
Keywords: Lung cancer; PD-L1; immunotherapy; lactate dehydrogenase (LDH); neutrophil-to-lymphocyte ratio (NLR).
2020 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-19-583). MB reports personal fees from Janssen-Cilag, Boehringer Ingelheim, Roche, non-financial support from Bristol-Myers Squibb, AstraZeneca/MedImmune, Pierre Fabre, Ipsen, outside the submitted work. DS reports personal fees and non-financial support from Astra Zeneca, Bristol Myers Squibb, personal fees from Lilly, non-financial support from Merck Sharp & Dohme, Roche outside the submitted work. Dr. Metro reports personal fees from Boehringher-Ingelheim outside the submitted work and serves as an unpaid editorial board member of Translational Lung Cancer Research from Jul 2019 to Jul 2021. ADT reports other from MSD outside the submitted work. AF reports personal fees from Roche, Pfizer, Astellas and Bristol-Myers Squibb outside the submitted work. MCG reports personal fees and other from Eli Lilly, personal fees from Boehringer Ingelheim, Otsuka Pharma, grants, personal fees and other from Astra Zeneca, Novartis, BMS, Roche, Pfizer, Celgene, personal fees from Incyte, Inivata, Takeda, grants and other from Tiziana Sciences, Clovis, Merck Serono, grants and personal fees from Bayer, grants, personal fees and other from MSD, grants and other from GlaxoSmithKline S.p.A., personal fees from Sanofi-Aventis, grants and other from Spectrum Pharmaceutcials, Blueprint Medicine, personal fees from Seattle Genetics, Daiichi Sankyo outside the submitted work. AA reports personal fees from BMS, Astrazeneca, Roche, Pfizer, MSD, Boehringer outside the submitted work. The other authors have no conflicts of interest to declare.
Figures



References
-
- Addeo A, Banna GL, Metro G, et al. Chemotherapy in Combination With Immune Checkpoint Inhibitors for the First-Line Treatment of Patients With Advanced Non-small Cell Lung Cancer: A Systematic Review and Literature-Based Meta-Analysis. Front Oncol 2019;9:264. 10.3389/fonc.2019.00264 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
