CAR-T Cells Targeting Epstein-Barr Virus gp350 Validated in a Humanized Mouse Model of EBV Infection and Lymphoproliferative Disease
- PMID: 32953984
- PMCID: PMC7479496
- DOI: 10.1016/j.omto.2020.08.005
CAR-T Cells Targeting Epstein-Barr Virus gp350 Validated in a Humanized Mouse Model of EBV Infection and Lymphoproliferative Disease
Abstract
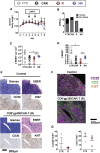
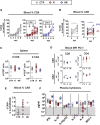
Epstein-Barr virus (EBV) is a latent and oncogenic human herpesvirus. Lytic viral protein expression plays an important role in EBV-associated malignancies. The EBV envelope glycoprotein 350 (gp350) is expressed abundantly during EBV lytic reactivation and sporadically on the surface of latently infected cells. Here we tested T cells expressing gp350-specific chimeric antigen receptors (CARs) containing scFvs derived from two novel gp350-binding, highly neutralizing monoclonal antibodies. The scFvs were fused to CD28/CD3ζ signaling domains in a retroviral vector. The produced gp350CAR-T cells specifically recognized and killed gp350+ 293T cells in vitro. The best-performing 7A1-gp350CAR-T cells were cytotoxic against the EBV+ B95-8 cell line, showing selectivity against gp350+ cells. Fully humanized Nod.Rag.Gamma mice transplanted with cord blood CD34+ cells and infected with the EBV/M81/fLuc lytic strain were monitored dynamically for viral spread. Infected mice recapitulated EBV-induced lymphoproliferation, tumor development, and systemic inflammation. We tested adoptive transfer of autologous CD8+gp350CAR-T cells administered protectively or therapeutically. After gp350CAR-T cell therapy, 75% of mice controlled or reduced EBV spread and showed lower frequencies of EBER+ B cell malignant lymphoproliferation, lack of tumor development, and reduced inflammation. In summary, CD8+gp350CAR-T cells showed proof-of-concept preclinical efficacy against impending EBV+ lymphoproliferation and lymphomagenesis.
Keywords: CAR-T cells; EBV; PTLD; adoptive T cell therapy; gp350; humanized mice; lymphoma; lymphoproliferation; lytic infection; transplantation.
© 2020 The Author(s).
Figures





Similar articles
-
GMP development and preclinical validation of CAR-T cells targeting a lytic EBV antigen for therapy of EBV-associated malignancies.Front Immunol. 2023 Feb 2;14:1103695. doi: 10.3389/fimmu.2023.1103695. eCollection 2023. Front Immunol. 2023. PMID: 36817460 Free PMC article.
-
Non-viral TRAC-knocked-in CD19KICAR-T and gp350KICAR-T cells tested against Burkitt lymphomas with type 1 or 2 EBV infection: In vivo cellular dynamics and potency.Front Immunol. 2023 Mar 24;14:1086433. doi: 10.3389/fimmu.2023.1086433. eCollection 2023. Front Immunol. 2023. PMID: 37033919 Free PMC article.
-
PD-1 Blockade Aggravates Epstein-Barr Virus+ Post-Transplant Lymphoproliferative Disorder in Humanized Mice Resulting in Central Nervous System Involvement and CD4+ T Cell Dysregulations.Front Oncol. 2021 Jan 12;10:614876. doi: 10.3389/fonc.2020.614876. eCollection 2020. Front Oncol. 2021. PMID: 33511078 Free PMC article.
-
Epstein Barr Virus: Development of Vaccines and Immune Cell Therapy for EBV-Associated Diseases.Front Immunol. 2021 Oct 8;12:734471. doi: 10.3389/fimmu.2021.734471. eCollection 2021. Front Immunol. 2021. PMID: 34691042 Free PMC article. Review.
-
Epstein-Barr virus vaccine development: a lytic and latent protein cocktail.Front Biosci. 2008 May 1;13:5916-27. doi: 10.2741/3126. Front Biosci. 2008. PMID: 18508632 Review.
Cited by
-
[Functional investigation of chimeric antigen receptor T cells targeting LMP1 antigen].Zhonghua Xue Ye Xue Za Zhi. 2022 Mar 14;43(3):229-234. doi: 10.3760/cma.j.issn.0253-2727.2022.03.008. Zhonghua Xue Ye Xue Za Zhi. 2022. PMID: 35405781 Free PMC article. Chinese.
-
Synergistic integration of mRNA-LNP with CAR-engineered immune cells: Pioneering progress in immunotherapy.Mol Ther. 2024 Nov 6;32(11):3772-3792. doi: 10.1016/j.ymthe.2024.09.019. Epub 2024 Sep 17. Mol Ther. 2024. PMID: 39295145 Review.
-
Research landmarks on the 60th anniversary of Epstein-Barr virus.Sci China Life Sci. 2025 Feb;68(2):354-380. doi: 10.1007/s11427-024-2766-0. Epub 2024 Nov 4. Sci China Life Sci. 2025. PMID: 39505801 Review.
-
The expanded application of CAR-T cell therapy for the treatment of multiple non-tumoral diseases.Protein Cell. 2024 Sep 1;15(9):633-641. doi: 10.1093/procel/pwad061. Protein Cell. 2024. PMID: 38146589 Free PMC article. No abstract available.
-
Leveraging oncovirus-derived antigen against the viral malignancies in adoptive cell therapies.Biomark Res. 2024 Jul 29;12(1):71. doi: 10.1186/s40364-024-00617-6. Biomark Res. 2024. PMID: 39075601 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

