Development and characterization of a photocurable alginate bioink for three-dimensional bioprinting
- PMID: 32954039
- PMCID: PMC7481104
- DOI: 10.18063/ijb.v5i2.189
Development and characterization of a photocurable alginate bioink for three-dimensional bioprinting
Erratum in
-
ERRATUM.Int J Bioprint. 2020 Sep 17;6(4):309. doi: 10.18063/ijb.v6i4.309. eCollection 2020. Int J Bioprint. 2020. PMID: 33102924 Free PMC article.
Abstract
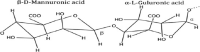
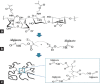
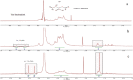
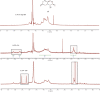
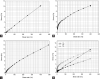
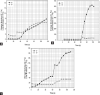
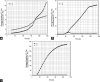
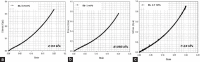
Alginate is a biocompatible material suitable for biomedical applications, which can be processed under mild conditions on irradiation. This paper investigates the preparation and the rheological behavior of different pre-polymerized and polymerized alginate methacrylate systems for three-dimensional photopolymerization bioprinting. The effect of the functionalization time on the mechanical, morphological, swelling, and degradation characteristics of cross-linked alginate hydrogel is also discussed. Alginate was chemically-modified with methacrylate groups and different reaction times considered. Photocurable alginate systems were prepared by dissolving functionalized alginate with 0.5- 1.5% w/v photoinitiator solutions and cross-linked by ultraviolet light (8 mW/cm2 for 8 minutes).
Keywords: 3D bioprinting; Alginate hydrogel; Functionalization; Photopolymerization; Rheology.
Copyright: © 2019 Mishbak, et al.
Figures











References
-
- Pereira R, Bártolo PJF. 3D Bioprinting of Photocrosslinkable Hydrogel Constructs. Appl Polym Sci. DOI 10.1002/app42458 2015;132(48):132A4.
-
- Zhou D, Yoshihiro I. Visible Light-curable Polymers for Biomedical Applications. Sci China Chem. 2014;57(4):510–21. DOI 10.1007/s11426-014-5069-z.
-
- Pereira RF, Sousa A, Barrias CC, et al. A Single-component Hydrogel Bioink for Bioprinting of Bioengineered 3D Constructs for Dermal Tissue Engineering. Mater Horiz. 2018;5(6):1100–11. DOI 10.1039/c8mh00525g.
-
- Vyas C, Pereira R, Huang B, et al. Engineering the Vasculature with Additive Manufacturing. Curr Opin Biomed Eng. 2017;2:1–13.
LinkOut - more resources
Full Text Sources
