Conditioned medium-electrospun fiber biomaterials for skin regeneration
- PMID: 32954054
- PMCID: PMC7481508
- DOI: 10.1016/j.bioactmat.2020.08.022
Conditioned medium-electrospun fiber biomaterials for skin regeneration
Erratum in
-
Corrigendum to 'Conditioned medium-electrospun fiber biomaterials for skin regeneration' [Bioact. Mater. 6 (2021) 361-374].Bioact Mater. 2021 Feb 15;6(9):2752-2753. doi: 10.1016/j.bioactmat.2021.02.001. eCollection 2021 Sep. Bioact Mater. 2021. PMID: 33665506 Free PMC article.
Abstract
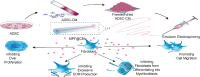
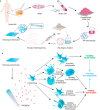
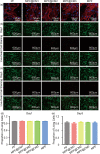
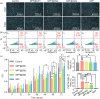
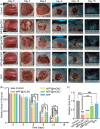
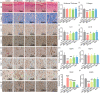
Conditioned medium (CM) contains variety of factors secreted by cells, which directly regulate cellular processes, showing tremendous potential in regenerative medicine. Here, for the first time, we proposed a novel regenerative therapy mediated by biodegradable micro-nano electrospun fibers loaded with highly active conditioned medium of adipose-derived stem cells (ADSC-CM). ADSC-CM was successfully loaded into the nanofibers with biological protection and controllable sustained-release properties by emulsion electrospinning and protein freeze-drying technologies. In vitro, ADSC-CM released by the fibers accelerated the migration rate of fibroblasts; inhibited the over proliferation of fibroblasts by inducing apoptosis and damaging cell membrane; in addition, ADSC-CM inhibited the transformation of fibroblasts into myofibroblasts and suppressed excessive production of extracellular matrix (ECM). In vivo, the application of CM-biomaterials significantly accelerated wound closure and improved regeneration outcome, showing superior pro-regenerative performance. This study pioneered the application of CM-biomaterials in regenerative medicine, and confirmed the practicability and significant biological effects of this innovative biomaterials.
Keywords: Biomaterials; Conditioned medium; Electrospun fiber; Regenerative medicine; Skin regeneration.
© 2020 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interests.
Figures


Similar articles
-
Galectin-1 from conditioned medium of three-dimensional culture of adipose-derived stem cells accelerates migration and proliferation of human keratinocytes and fibroblasts.Wound Repair Regen. 2018 Dec;26 Suppl 1:S9-S18. doi: 10.1111/wrr.12579. Epub 2017 Nov 8. Wound Repair Regen. 2018. PMID: 28857355
-
Extracellular matrix/stromal vascular fraction gel conditioned medium accelerates wound healing in a murine model.Wound Repair Regen. 2017 Nov;25(6):923-932. doi: 10.1111/wrr.12602. Epub 2018 Feb 6. Wound Repair Regen. 2017. PMID: 29240284
-
Ell3 Modulates the Wound Healing Activity of Conditioned Medium of Adipose-derived Stem Cells.Dev Reprod. 2017 Sep;21(3):335-342. doi: 10.12717/DR.2017.21.3.335. Epub 2017 Sep 30. Dev Reprod. 2017. PMID: 29082349 Free PMC article.
-
Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: Implications for scaffold design and performance.Acta Biomater. 2017 Mar 1;50:41-55. doi: 10.1016/j.actbio.2016.12.034. Epub 2016 Dec 21. Acta Biomater. 2017. PMID: 28011142 Review.
-
Electrospun Fibers for Recruitment and Differentiation of Stem Cells in Regenerative Medicine.Biotechnol J. 2017 Dec;12(12). doi: 10.1002/biot.201700263. Epub 2017 Oct 30. Biotechnol J. 2017. PMID: 28980771 Review.
Cited by
-
Cell secretomes for wound healing and tissue regeneration: Next generation acellular based tissue engineered products.J Tissue Eng. 2022 Jul 28;13:20417314221114273. doi: 10.1177/20417314221114273. eCollection 2022 Jan-Dec. J Tissue Eng. 2022. PMID: 35923177 Free PMC article. Review.
-
The Effect of Physiological Incubation on the Properties of Elastic Magnetic Composites for Soft Biomedical Sensors.Sensors (Basel). 2021 Oct 27;21(21):7122. doi: 10.3390/s21217122. Sensors (Basel). 2021. PMID: 34770427 Free PMC article.
-
Antibacterial 3D-Printed Silver Nanoparticle/Poly Lactic-Co-Glycolic Acid (PLGA) Scaffolds for Bone Tissue Engineering.Materials (Basel). 2023 May 23;16(11):3895. doi: 10.3390/ma16113895. Materials (Basel). 2023. PMID: 37297029 Free PMC article.
-
Natural Compounds and Biomimetic Engineering to Influence Fibroblast Behavior in Wound Healing.Int J Mol Sci. 2024 Mar 14;25(6):3274. doi: 10.3390/ijms25063274. Int J Mol Sci. 2024. PMID: 38542247 Free PMC article. Review.
-
Amnion-Derived Teno-Inductive Secretomes: A Novel Approach to Foster Tendon Differentiation and Regeneration in an Ovine Model.Front Bioeng Biotechnol. 2021 Mar 11;9:649288. doi: 10.3389/fbioe.2021.649288. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33777919 Free PMC article.
References
-
- Falanga V., Iwamoto S., Chartier M., Yufit T., Butmarc J., Kouttab N., Shrayer D., Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. - DOI - PubMed
-
- Bey E., Prat M., Duhamel P., Benderitter M., Brachet M., Trompier F., Battaglini P., Ernou I., Boutin L., Gourven M., Tissedre F., Créa S., Mansour C.A., de Revel T., Carsin H., Gourmelon P., Lataillade J.J. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 2010;18:50–58. doi: 10.1111/j.1524-475X.2009.00562.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

