Efficient Lone-Pair-Driven Luminescence: Structure-Property Relationships in Emissive 5s2 Metal Halides
- PMID: 32954359
- PMCID: PMC7491574
- DOI: 10.1021/acsmaterialslett.0c00211
Efficient Lone-Pair-Driven Luminescence: Structure-Property Relationships in Emissive 5s2 Metal Halides
Abstract
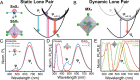
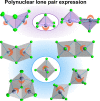
Low-dimensional metal halides have been the focus of intense investigations in recent years following the success of hybrid lead halide perovskites as optoelectronic materials. In particular, the light emission of low-dimensional halides based on the 5s2 cations Sn2+ and Sb3+ has found utility in a variety of applications complementary to those of the three-dimensional halide perovskites because of its unusual properties such as broadband character and highly temperature-dependent lifetime. These properties derive from the exceptional chemistry of the 5s2 lone pair, but the terminology and explanations given for such emission vary widely, hampering efforts to build a cohesive understanding of these materials that would lead to the development of efficient optoelectronic devices. In this Perspective, we provide a structural overview of these materials with a focus on the dynamics driven by the stereoactivity of the 5s2 lone pair to identify the structural features that enable strong emission. We unite the different theoretical models that have been able to explain the success of these bright 5s2 emission centers into a cohesive framework, which is then applied to the array of compounds recently developed by our group and other researchers, demonstrating its utility and generating a holistic picture of the field from the point of view of a materials chemist. We highlight those state-of-the-art materials and applications that demonstrate the unique capabilities of these versatile emissive centers and identify promising future directions in the field of low-dimensional 5s2 metal halides.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Yaffe O.; Guo Y.; Tan L. Z.; Egger D. A.; Hull T.; Stoumpos C. C.; Zheng F.; Heinz T. F.; Kronik L.; Kanatzidis M. G.; Owen J. S.; Rappe A. M.; Pimenta M. A.; Brus L. E. Local Polar Fluctuations in Lead Halide Perovskite Crystals. Phys. Rev. Lett. 2017, 118, 136001.10.1103/PhysRevLett.118.136001. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
