C-Cbl regulates c-MPL receptor trafficking and its internalization
- PMID: 32954656
- PMCID: PMC7687000
- DOI: 10.1111/jcmm.15785
C-Cbl regulates c-MPL receptor trafficking and its internalization
Abstract
Thrombocyte formation from megakaryocyte and their progenitor cells is tightly regulated by thrombopoietin (TPO) and its receptor c-MPL, thereby maintaining physiological functionality and numbers of circulating platelets. In patients, dysfunction of this regulation could cause thrombocytopenia or myeloproliferative syndromes. Since regulation of this pathway is still not completely understood, we investigated the role of the ubiquitin ligase c-Cbl which was previously shown to negatively regulated c-MPL signalling. We developed a new conditional mouse model using c-Cblfl/fl Pf4Cre mice and demonstrated that platelet-specific knockout of c-Cbl led to severe microthrombocytosis and impaired uptake of TPO and c-MPL receptor internalization. Furthermore, we characterized a constitutive STAT5 activation c-Cbl KO platelets. This study identified c-Cbl as a potential player in causing megakaryocytic and thrombocytic disorders.
Keywords: C-Cbl; c-MPL; megakaryocytes; platelets; thrombocytosis.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures

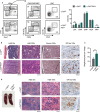
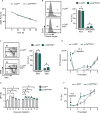
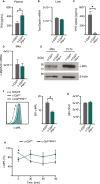

References
-
- Kaushansky K. Thrombopoietin and the hematopoietic stem cell. Ann N Y Acad Sci. 2005;1044:139‐141. - PubMed
-
- Kaushansky K, Drachman JG. The molecular and cellular biology of thrombopoietin: the primary regulator of platelet production. Oncogene. 2002;21(21):3359‐3367. - PubMed
-
- Steinberg O, Gilad G, Dgany O, et al. Congenital amegakaryocytic thrombocytopenia‐3 novel c‐MPL mutations and their phenotypic correlations. J Pediatr Hematol Oncol. 2007;29(12):822‐825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

