Glucagon-like peptide-1 receptor agonists as neuroprotective agents for ischemic stroke: a systematic scoping review
- PMID: 32954901
- PMCID: PMC7747170
- DOI: 10.1177/0271678X20952011
Glucagon-like peptide-1 receptor agonists as neuroprotective agents for ischemic stroke: a systematic scoping review
Abstract
Stroke mortality and morbidity is expected to rise. Despite considerable recent advances within acute ischemic stroke treatment, scope remains for development of widely applicable neuroprotective agents. Glucagon-like peptide-1 receptor agonists (GLP-1RAs), originally licensed for the management of Type 2 Diabetes Mellitus, have demonstrated pre-clinical neuroprotective efficacy in a range of neurodegenerative conditions. This systematic scoping review reports the pre-clinical basis of GLP-1RAs as neuroprotective agents in acute ischemic stroke and their translation into clinical trials. We included 35 pre-clinical studies, 11 retrospective database studies, 7 cardiovascular outcome trials and 4 prospective clinical studies. Pre-clinical neuroprotection was demonstrated in normoglycemic models when administration was delayed by up to 24 h following stroke induction. Outcomes included reduced infarct volume, apoptosis, oxidative stress and inflammation alongside increased neurogenesis, angiogenesis and cerebral blood flow. Improved neurological function and a trend towards increased survival were also reported. Cardiovascular outcomes trials reported a significant reduction in stroke incidence with semaglutide and dulaglutide. Retrospective database studies show a trend towards neuroprotection. Prospective interventional clinical trials are on-going, but initial indicators of safety and tolerability are favourable. Ultimately, we propose that repurposing GLP-1RAs is potentially advantageous but appropriately designed trials are needed to determine clinical efficacy and cost-effectiveness.
Keywords: Acute stroke; animal models; clinical trials; neuroprotection; reperfusion.
Conflict of interest statement
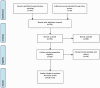
Figures

References
-
- Feigin VL, Norrving B, Mensah GA.Global burden of stroke. Circ Res 2017; 120: 439–448. - PubMed
-
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

