Degeneration of Bioprosthetic Heart Valves: Update 2020
- PMID: 32954917
- PMCID: PMC7792365
- DOI: 10.1161/JAHA.120.018506
Degeneration of Bioprosthetic Heart Valves: Update 2020
Abstract
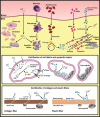
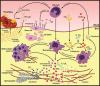
The implantation of bioprosthetic heart valves (BHVs) is increasingly becoming the treatment of choice in patients requiring heart valve replacement surgery. Unlike mechanical heart valves, BHVs are less thrombogenic and exhibit superior hemodynamic properties. However, BHVs are prone to structural valve degeneration (SVD), an unavoidable condition limiting graft durability. Mechanisms underlying SVD are incompletely understood, and early concepts suggesting the purely degenerative nature of this process are now considered oversimplified. Recent studies implicate the host immune response as a major modality of SVD pathogenesis, manifested by a combination of processes phenocopying the long-term transplant rejection, atherosclerosis, and calcification of native aortic valves. In this review, we summarize and critically analyze relevant studies on (1) SVD triggers and pathogenesis, (2) current approaches to protect BHVs from calcification, (3) obtaining low immunogenic BHV tissue from genetically modified animals, and (4) potential strategies for SVD prevention in the clinical setting.
Keywords: bioprosthesis; calcification; genetically modified animals; immune rejection; inflammation; structural valve degeneration; valve replacement.
Conflict of interest statement
None.
Figures



References
-
- Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. - PubMed
-
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e1159–e1195. - PubMed
-
- Head SJ, Çelik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017;38:2183–2191. - PubMed
-
- Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, Lancellotti P, Sondergaard L, Ludman PF, Tamburino C, et al. Standardized definitions of structural deterioration and valve failure in assessing long‐term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2017;38:3382–3390. - PubMed
-
- Dvir D, Bourguignon T, Otto CM, Hahn RT, Rosenhek R, Webb JG, Treede H, Sarano ME, Feldman T, Wijeysundera HC, et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137:388–399. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

