Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE)
- PMID: 32954927
- PMCID: PMC7768339
- DOI: 10.1200/JCO.20.02514
Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE)
Abstract
Purpose: Many patients with HR+, HER2- early breast cancer (EBC) will not experience recurrence or have distant recurrence with currently available standard therapies. However, up to 30% of patients with high-risk clinical and/or pathologic features may experience distant recurrence, many in the first few years. Superior treatment options are needed to prevent early recurrence and development of metastases for this group of patients. Abemaciclib is an oral, continuously dosed, CDK4/6 inhibitor approved for HR+, HER2- advanced breast cancer (ABC). Efficacy and safety of abemaciclib in ABC supported evaluation in the adjuvant setting.
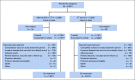
Methods: This open-label, phase III study included patients with HR+, HER2-, high-risk EBC, who had surgery and, as indicated, radiotherapy and/or adjuvant/neoadjuvant chemotherapy. Patients with four or more positive nodes, or one to three nodes and either tumor size ≥ 5 cm, histologic grade 3, or central Ki-67 ≥ 20%, were eligible and randomly assigned (1:1) to standard-of-care adjuvant endocrine therapy (ET) with or without abemaciclib (150 mg twice daily for 2 years). The primary end point was invasive disease-free survival (IDFS), and secondary end points included distant relapse-free survival, overall survival, and safety.
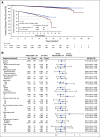
Results: At a preplanned efficacy interim analysis, among 5,637 randomly assigned patients, 323 IDFS events were observed in the intent-to-treat population. Abemaciclib plus ET demonstrated superior IDFS versus ET alone (P = .01; hazard ratio, 0.75; 95% CI, 0.60 to 0.93), with 2-year IDFS rates of 92.2% versus 88.7%, respectively. Safety data were consistent with the known safety profile of abemaciclib.
Conclusion: Abemaciclib when combined with ET is the first CDK4/6 inhibitor to demonstrate a significant improvement in IDFS in patients with HR+, HER2- node-positive EBC at high risk of early recurrence.
Trial registration: ClinicalTrials.gov NCT03155997.
Figures

Comment in
-
CDK4/6 Inhibition in Early-Stage Breast Cancer: The New Standard?J Clin Oncol. 2020 Dec 1;38(34):3977-3979. doi: 10.1200/JCO.20.02688. Epub 2020 Sep 20. J Clin Oncol. 2020. PMID: 32954928 No abstract available.
-
CDK4/6 inhibitors for HR+HER2- early stage breast cancer - when to escalate treatment?Nat Rev Clin Oncol. 2021 Feb;18(2):67-68. doi: 10.1038/s41571-020-00453-1. Nat Rev Clin Oncol. 2021. PMID: 33235325 No abstract available.
-
Reply to K. Hashimoto and A. Shimomura.J Clin Oncol. 2021 May 1;39(13):1507-1508. doi: 10.1200/JCO.20.03477. Epub 2021 Feb 25. J Clin Oncol. 2021. PMID: 33630658 No abstract available.
-
Regarding the Addition of CDK4/6 Inhibitor to Postoperative Endocrine Therapy in Patients With HR-Positive HER2-Negative High-Risk Breast Cancer.J Clin Oncol. 2021 May 1;39(13):1506-1507. doi: 10.1200/JCO.20.03215. Epub 2021 Feb 25. J Clin Oncol. 2021. PMID: 33630669 No abstract available.
-
Adjuvant Abemaciclib Plus Endocrine Therapy in the Treatment of High-Risk Early Breast Cancer: ASCO Guideline Rapid Recommendation Update Q and A.JCO Oncol Pract. 2022 Jul;18(7):516-519. doi: 10.1200/OP.22.00140. Epub 2022 Apr 4. JCO Oncol Pract. 2022. PMID: 35377771 No abstract available.
References
-
- Cardoso F Spence D Mertz S, et al. : Global analysis of advanced/metastatic breast cancer: Decade report (2005-2015). Breast 39:131-138, 2018 - PubMed
-
- Senkus E Kyriakides S Ohno S, et al. : Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v8-v30, 2015. (suppl 5) - PubMed
-
- National Comprehensive Cancer Network: Breast Cancer (version 4.2020). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
-
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) : Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 386:1341-1352, 2015 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

