Rezafungin Versus Caspofungin in a Phase 2, Randomized, Double-blind Study for the Treatment of Candidemia and Invasive Candidiasis: The STRIVE Trial
- PMID: 32955088
- PMCID: PMC8662762
- DOI: 10.1093/cid/ciaa1380
Rezafungin Versus Caspofungin in a Phase 2, Randomized, Double-blind Study for the Treatment of Candidemia and Invasive Candidiasis: The STRIVE Trial
Erratum in
-
Erratum to: Rezafungin Versus Caspofungin in a Phase 2, Randomized, Double-Blind Study for the Treatment of Candidemia and Invasive Candidiasis: The STRIVE Trial.Clin Infect Dis. 2021 Aug 2;73(3):561-562. doi: 10.1093/cid/ciab402. Clin Infect Dis. 2021. PMID: 34165553 Free PMC article. No abstract available.
Abstract
Background: Rezafungin (RZF) is a novel echinocandin exhibiting distinctive pharmacokinetics/pharmacodynamics. STRIVE was a phase 2, double-blind, randomized trial designed to compare the safety and efficacy of RZF once weekly (QWk) to caspofungin (CAS) once daily for treatment of candidemia and/or invasive candidiasis (IC).
Methods: Adults with systemic signs and mycological confirmation of candidemia and/or IC were randomized to RZF 400 mg QWk (400 mg), RZF 400 mg on week 1 then 200 mg QWk (400/200 mg), or CAS 70 mg as a loading dose followed by 50 mg daily for ≤4 weeks. Efficacy assessments included overall cure (resolution of signs of candidemia/IC + mycological eradication) at day 14 (primary endpoint), investigator-assessed clinical response at day 14, and 30-day all-cause mortality (ACM) (secondary endpoints), and time to negative blood culture. Safety was evaluated by adverse events and ACM through follow-up.
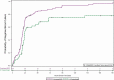
Results: Of 207 patients enrolled, 183 were in the microbiological intent-to-treat population (~21% IC). Overall cure rates were 60.5% (46/76) for RZF 400 mg, 76.1% (35/46) for RZF 400/200 mg, and 67.2% (41/61) for CAS; investigator-assessed clinical cure rates were 69.7% (53/76), 80.4% (37/46), and 70.5% (43/61), respectively. In total, 30-day ACM was 15.8% for RZF 400 mg, 4.4% for RZF 400/200 mg, and 13.1% for CAS. Candidemia was cleared in 19.5 and 22.8 hours in RZF and CAS patients, respectively. No concerning safety trends were observed; ACM through follow-up was 15.2% (21/138) for RZF and 18.8% (13/69) for CAS.
Conclusions: RZF was safe and efficacious in the treatment of candidemia and/or IC.
Clinical trials registration: NCT02734862.
Keywords: candidemia; echinocandins; rezafungin; systemic antifungal therapy.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

References
-
- Andes DR, Safdar N, Baddley JW, et al. ; Mycoses Study Group . Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012; 54:1110–22. - PubMed
-
- Kullberg BJ, Viscoli C, Pappas PG, et al. . Isavuconazole versus caspofungin in the treatment of candidemia and other invasive Candida infections: the ACTIVE trial. Clin Infect Dis 2019; 68:1981–9. - PubMed
-
- Cornely OA, Bassetti M, Calandra T, et al. ; ESCMID Fungal Infection Study Group . ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 2012; 18(Suppl 7):19–37. - PubMed

