Chronic postnatal chemogenetic activation of forebrain excitatory neurons evokes persistent changes in mood behavior
- PMID: 32955432
- PMCID: PMC7652419
- DOI: 10.7554/eLife.56171
Chronic postnatal chemogenetic activation of forebrain excitatory neurons evokes persistent changes in mood behavior
Abstract
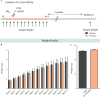
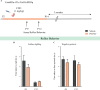
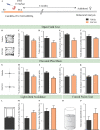
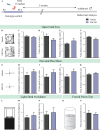
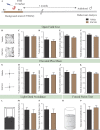
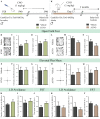
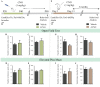
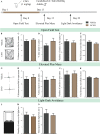
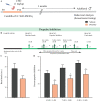
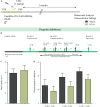
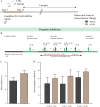
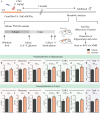
Early adversity is a risk factor for the development of adult psychopathology. Common across multiple rodent models of early adversity is increased signaling via forebrain Gq-coupled neurotransmitter receptors. We addressed whether enhanced Gq-mediated signaling in forebrain excitatory neurons during postnatal life can evoke persistent mood-related behavioral changes. Excitatory hM3Dq DREADD-mediated chemogenetic activation of forebrain excitatory neurons during postnatal life (P2-14), but not in juvenile or adult windows, increased anxiety-, despair-, and schizophrenia-like behavior in adulthood. This was accompanied by an enhanced metabolic rate of cortical and hippocampal glutamatergic and GABAergic neurons. Furthermore, we observed reduced activity and plasticity-associated marker expression, and perturbed excitatory/inhibitory currents in the hippocampus. These results indicate that Gq-signaling-mediated activation of forebrain excitatory neurons during the critical postnatal window is sufficient to program altered mood-related behavior, as well as functional changes in forebrain glutamate and GABA systems, recapitulating aspects of the consequences of early adversity.
Keywords: DREADD; anxiety; despair; early stress; mouse; neuroscience; schizophrenia.
Plain language summary
Stress and adversity in early childhood can have long-lasting effects, predisposing people to mental illness and mood disorders in adult life. The weeks immediately before and after birth are critical for establishing key networks of neurons in the brain. Therefore, any disruption to these neural circuits during this time can be detrimental to emotional development. However, it is still unclear which cellular mechanisms cause these lasting changes in behavior. Studies in animals suggest that these long-term effects could result from abnormalities in a few signaling pathways in the brain. For example, it has been proposed that overstimulating the cells that activate circuits in the forebrain – also known as excitatory neurons – may contribute to the behavioral changes that persist into adulthood. To test this theory, Pati et al. used genetic engineering to modulate a signaling pathway in male mice, which is known to stimulate excitatory neurons in the forebrain. The experiments showed that prolonged activation of excitatory neurons in the first two weeks after birth resulted in anxious and despair-like behaviors as the animals aged. The mice also displayed discrepancies in how they responded to certain external sensory information, which is a hallmark of schizophrenia-like behavior. However, engineering the same changes in adolescent and adult mice had no effect on their mood-related behaviors. This animal study reinforces just how critical the first few weeks of life are for optimal brain development. It provides an insight into a possible mechanism of how disruption during this time could alter emotional behavior. The findings are also relevant to psychiatrists interested in the underlying causes of mental illness after early childhood adversity.
© 2020, Pati et al.
Conflict of interest statement
SP, KS, SS, PT, PC, VV, SM, DK, SS, JC, AP No competing interests declared, VV Reviewing editor, eLife
Figures
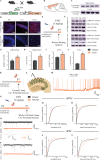
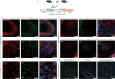


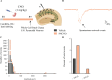
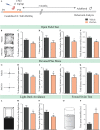






References
-
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. - DOI - PMC - PubMed
-
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

