Site-specific effects of neurosteroids on GABAA receptor activation and desensitization
- PMID: 32955433
- PMCID: PMC7532004
- DOI: 10.7554/eLife.55331
Site-specific effects of neurosteroids on GABAA receptor activation and desensitization
Abstract
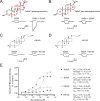
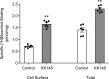
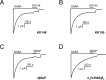
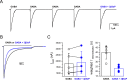
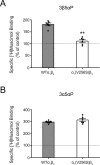
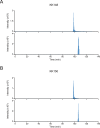
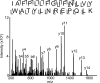
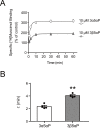
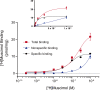
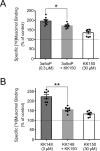
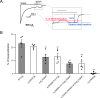
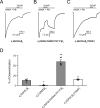
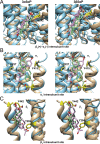
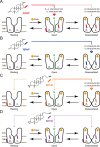
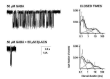
This study examines how site-specific binding to three identified neurosteroid-binding sites in the α1β3 GABAA receptor (GABAAR) contributes to neurosteroid allosteric modulation. We found that the potentiating neurosteroid, allopregnanolone, but not its inhibitory 3β-epimer epi-allopregnanolone, binds to the canonical β3(+)-α1(-) intersubunit site that mediates receptor activation by neurosteroids. In contrast, both allopregnanolone and epi-allopregnanolone bind to intrasubunit sites in the β3 subunit, promoting receptor desensitization and the α1 subunit promoting effects that vary between neurosteroids. Two neurosteroid analogues with diazirine moieties replacing the 3-hydroxyl (KK148 and KK150) bind to all three sites, but do not potentiate GABAAR currents. KK148 is a desensitizing agent, whereas KK150 is devoid of allosteric activity. These compounds provide potential chemical scaffolds for neurosteroid antagonists. Collectively, these data show that differential occupancy and efficacy at three discrete neurosteroid-binding sites determine whether a neurosteroid has potentiating, inhibitory, or competitive antagonist activity on GABAARs.
Keywords: desensitization; gabaa receptor; human; molecular biophysics; neurosteroids; photoaffinity labeling; radioligand binding; site-directed mutagenesis; structural biology.
© 2020, Sugasawa et al.
Conflict of interest statement
YS, WC, JB, ZC, LW, AG, SP, TS, KK, DR, DC, GA, AE No competing interests declared
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

