Whole Animal Imaging of Drosophila melanogaster using Microcomputed Tomography
- PMID: 32955492
- PMCID: PMC7891681
- DOI: 10.3791/61515
Whole Animal Imaging of Drosophila melanogaster using Microcomputed Tomography
Abstract
Biomedical imaging tools permit investigation of molecular mechanisms across spatial scales, from genes to organisms. Drosophila melanogaster, a well-characterized model organism, has benefited from the use of light and electron microscopy to understand gene function at the level of cells and tissues. The application of imaging platforms that allow for an understanding of gene function at the level of the entire intact organism would further enhance our knowledge of genetic mechanisms. Here a whole animal imaging method is presented that outlines the steps needed to visualize Drosophila at any developmental stage using microcomputed tomography (µ-CT). The advantages of µ-CT include commercially available instrumentation and minimal hands-on time to produce accurate 3D information at micron-level resolution without the need for tissue dissection or clearing methods. Paired with software that accelerate image analysis and 3D rendering, detailed morphometric analysis of any tissue or organ system can be performed to better understand mechanisms of development, physiology, and anatomy for both descriptive and hypothesis testing studies. By utilizing an imaging workflow that incorporates the use of electron microscopy, light microscopy, and µ-CT, a thorough evaluation of gene function can be performed, thus furthering the usefulness of this powerful model organism.
Conflict of interest statement
Disclosures
The author declares no competing or financial interests.
Figures

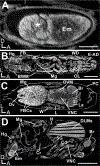

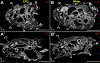
References
-
- Rahman IA, Smith SY Virtual paleontology: computer-aided analysis of fossil form and function. Journal of Paleontology. 88 (04), 633–635 (2014).
-
- Carlson WD Three-dimensional imaging of earth and planetary materials. Earth and Planetary Science Letters. 249 (3–4), 133–147 (2006).
-
- Abel RL et al. Digital preservation and dissemination of ancient lithic technology with modern micro-CT. Computers & Graphics. 35 (4), 878–884 (2011).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
