Enabling Real-Time Compensation in Fast Photochemical Oxidations of Proteins for the Determination of Protein Topography Changes
- PMID: 32955502
- PMCID: PMC7695538
- DOI: 10.3791/61580
Enabling Real-Time Compensation in Fast Photochemical Oxidations of Proteins for the Determination of Protein Topography Changes
Abstract
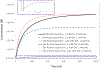
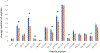
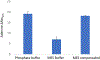
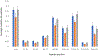
Fast photochemical oxidation of proteins (FPOP) is a mass spectrometry-based structural biology technique that probes the solvent-accessible surface area of proteins. This technique relies on the reaction of amino acid side chains with hydroxyl radicals freely diffusing in solution. FPOP generates these radicals in situ by laser photolysis of hydrogen peroxide, creating a burst of hydroxyl radicals that is depleted on the order of a microsecond. When these hydroxyl radicals react with a solvent-accessible amino acid side chain, the reaction products exhibit a mass shift that can be measured and quantified by mass spectrometry. Since the rate of reaction of an amino acid depends in part on the average solvent accessible surface of that amino acid, measured changes in the amount of oxidation of a given region of a protein can be directly correlated to changes in the solvent accessibility of that region between different conformations (e.g., ligand-bound versus ligand-free, monomer vs. aggregate, etc.) FPOP has been applied in a number of problems in biology, including protein-protein interactions, protein conformational changes, and protein-ligand binding. As the available concentration of hydroxyl radicals varies based on many experimental conditions in the FPOP experiment, it is important to monitor the effective radical dose to which the protein analyte is exposed. This monitoring is efficiently achieved by incorporating an inline dosimeter to measure the signal from the FPOP reaction, with laser fluence adjusted in real-time to achieve the desired amount of oxidation. With this compensation, changes in protein topography reflecting conformational changes, ligand-binding surfaces, and/or protein-protein interaction interfaces can be determined in heterogeneous samples using relatively low sample amounts.
Figures



References
-
- Buxton GV, Greenstock CL, Helman WP & Ross AB Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. Journal of Physical and Chemical Reference Data. 17 (2), 513–886, doi:10.1063/1.555805 (1988). - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
