Trends in Reperfusion Therapy for In-Hospital Ischemic Stroke in the Endovascular Therapy Era
- PMID: 32955582
- PMCID: PMC7506601
- DOI: 10.1001/jamaneurol.2020.3362
Trends in Reperfusion Therapy for In-Hospital Ischemic Stroke in the Endovascular Therapy Era
Abstract
Importance: A significant proportion of acute ischemic strokes occur while patients are hospitalized. Limited contemporary data exist on the utilization rates of intravenous thrombolysis or endovascular therapy for in-hospital stroke.
Objective: To use a national registry to examine temporal trends in the use of intravenous and endovascular reperfusion therapies for treatment of in-hospital stroke.
Design, setting, and participants: This retrospective cohort study analyzed data from 267 956 patients who underwent reperfusion therapy for stroke with in-hospital or out-of-hospital onset reported in the Get With the Guidelines-Stroke national registry from January 2008 to September 2018.
Exposures: In-hospital onset vs out-of-hospital onset of stroke symptoms.
Main outcomes and measures: Temporal trends in the use of reperfusion therapy, process measures of quality, and the association between functional outcomes and key patient characteristics, comorbidities, and treatments.
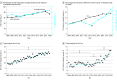
Results: Of 67 493 patients with in-hospital stroke onset, this study observed increased rates of vascular risk factors (standardized mean difference >10%) but no significant differences in age or sex in patients undergoing intravenous thrombolysis only (mean [interquartile range {IQR}] age, 72 [80-62] y; 53.2% female) or those undergoing endovascular therapy (mean [IQR] age, 69 [59-79] y; 49.8% female). Of these patients, 10 481 (15.5%) received intravenous thrombolysis and 2494 (3.7%) underwent endovascular therapy. Compared with 2008, in 2018 the proportion of in-hospital stroke among all stroke hospital discharges was higher (3.5% vs 2.7%; P < .001), as was use of intravenous thrombolysis (19.1% vs 9.1%; P < .001) and endovascular therapy (6.4% vs 2.5%; P < .001) in patients with in-hospital stroke, with a significant increase in endovascular therapy in mid-2015 (P < .001). Compared with patients who received intravenous thrombolysis for out-of-hospital stroke onset, those with in-hospital onset were associated with longer median (IQR) times from stroke recognition to cranial imaging (33 [18-60] vs 16 [9-26] minutes; P < .001) and to thrombolysis bolus (81 [52-125] vs 60 [45-84] minutes; P < .001). In adjusted analyses, patients with in-hospital stroke onset who were treated with intravenous thrombolysis were less likely to ambulate independently at discharge (adjusted odds ratio, 0.78; 95% CI, 0.74-0.82; P < .001) and were more likely to die or to be discharged to hospice (adjusted odds ratio, 1.39; 95% CI, 1.29-1.50; P < .001) than patients with out-of-hospital onset who also received intravenous thrombolysis treatment. Comparisons among patients treated with endovascular therapy yielded similar findings.
Conclusions and relevance: In this cohort study, in-hospital stroke onset was increasingly reported and treated with reperfusion therapy. Compared with out-of-hospital stroke onset, in-hospital onset was associated with longer delays to reperfusion and worse functional outcomes, highlighting opportunities to further care for patients with in-hospital stroke onset.
Conflict of interest statement
Figures
Comment in
-
In-Hospital Acute Strokes-Opportunities to Optimize Care and Improve Outcomes.JAMA Neurol. 2020 Dec 1;77(12):1482-1483. doi: 10.1001/jamaneurol.2020.3368. JAMA Neurol. 2020. PMID: 32955583 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

