Mechanical loading of tissue engineered skeletal muscle prevents dexamethasone induced myotube atrophy
- PMID: 32955689
- PMCID: PMC8332579
- DOI: 10.1007/s10974-020-09589-0
Mechanical loading of tissue engineered skeletal muscle prevents dexamethasone induced myotube atrophy
Abstract
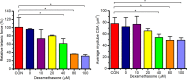
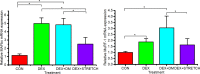
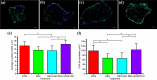
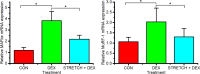
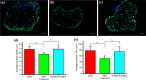
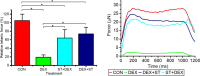
Skeletal muscle atrophy as a consequence of acute and chronic illness, immobilisation, muscular dystrophies and aging, leads to severe muscle weakness, inactivity and increased mortality. Mechanical loading is thought to be the primary driver for skeletal muscle hypertrophy, however the extent to which mechanical loading can offset muscle catabolism has not been thoroughly explored. In vitro 3D-models of skeletal muscle provide a controllable, high throughput environment and mitigating many of the ethical and methodological constraints present during in vivo experimentation. This work aimed to determine if mechanical loading would offset dexamethasone (DEX) induced skeletal muscle atrophy, in muscle engineered using the C2C12 murine cell line. Mechanical loading successfully offset myotube atrophy and functional degeneration associated with DEX regardless of whether the loading occurred before or after 24 h of DEX treatment. Furthermore, mechanical load prevented increases in MuRF-1 and MAFbx mRNA expression, critical regulators of muscle atrophy. Overall, we demonstrate the application of tissue engineered muscle to study skeletal muscle health and disease, offering great potential for future use to better understand treatment modalities for skeletal muscle atrophy.
Keywords: C2C12; Dexamethasone; Hypertrophy; Myotubes; Skeletal muscle; Ubiquitin–proteasome.
© 2020. The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Pyropia yezoensis Protein Prevents Dexamethasone-Induced Myotube Atrophy in C2C12 Myotubes.Mar Drugs. 2018 Dec 8;16(12):497. doi: 10.3390/md16120497. Mar Drugs. 2018. PMID: 30544821 Free PMC article.
-
Dehydroandrographolide succinate attenuates dexamethasone-induced skeletal muscle atrophy by regulating Akt/GSK3β and MuRF-1 pathways.Eur J Pharmacol. 2025 Mar 5;990:177265. doi: 10.1016/j.ejphar.2025.177265. Epub 2025 Jan 10. Eur J Pharmacol. 2025. PMID: 39800251
-
Sulforaphane prevents dexamethasone-induced muscle atrophy via regulation of the Akt/Foxo1 axis in C2C12 myotubes.Biomed Pharmacother. 2017 Nov;95:1486-1492. doi: 10.1016/j.biopha.2017.09.002. Epub 2017 Sep 21. Biomed Pharmacother. 2017. PMID: 28946211
-
Protective Effect of Pyropia yezoensis Peptide on Dexamethasone-Induced Myotube Atrophy in C2C12 Myotubes.Mar Drugs. 2019 May 11;17(5):284. doi: 10.3390/md17050284. Mar Drugs. 2019. PMID: 31083497 Free PMC article.
-
Neuron-derived neurotrophic factor protects against dexamethasone-induced skeletal muscle atrophy.Biochem Biophys Res Commun. 2022 Feb 19;593:5-12. doi: 10.1016/j.bbrc.2022.01.028. Epub 2022 Jan 13. Biochem Biophys Res Commun. 2022. PMID: 35051783
Cited by
-
Nerve function restoration following targeted muscle reinnervation after varying delayed periods.Neural Regen Res. 2023 Dec;18(12):2762-2766. doi: 10.4103/1673-5374.373659. Neural Regen Res. 2023. PMID: 37449642 Free PMC article.
-
Contractile force assessment methods for in vitro skeletal muscle tissues.Elife. 2022 May 23;11:e77204. doi: 10.7554/eLife.77204. Elife. 2022. PMID: 35604384 Free PMC article. Review.
-
Molecular and Biomechanical Adaptations to Mechanical Stretch in Cultured Myotubes.Front Physiol. 2021 Aug 2;12:689492. doi: 10.3389/fphys.2021.689492. eCollection 2021. Front Physiol. 2021. PMID: 34408658 Free PMC article. Review.
-
Manual Therapy Exerts Local Anti-Inflammatory Effects Through Neutrophil Clearance.J Immunol Res. 2024 Nov 5;2024:5556042. doi: 10.1155/2024/5556042. eCollection 2024. J Immunol Res. 2024. PMID: 39534554 Free PMC article.
-
Ubiquitin Ligases at the Heart of Skeletal Muscle Atrophy Control.Molecules. 2021 Jan 14;26(2):407. doi: 10.3390/molecules26020407. Molecules. 2021. PMID: 33466753 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

