Health care-related time costs in patients with metastatic breast cancer
- PMID: 32955793
- PMCID: PMC7666754
- DOI: 10.1002/cam4.3461
Health care-related time costs in patients with metastatic breast cancer
Abstract
Background: Burdens related to time spent receiving cancer care may be substantial for patients with incurable, life-limiting cancers such as metastatic breast cancer (MBC). Estimates of time spent on health care are needed to inform treatment-related decision-making.
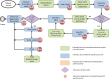
Methods: Estimates of time spent receiving cancer-related health care in the initial 3 months of treatment for patients with MBC were calculated using the following data sources: (a) direct observations from a time-in-motion quality improvement evaluation (process mapping); (b) cross-sectional patient surveys; and (c) administrative claims. Average ambulatory, inpatient, and total health care time were calculated for specific treatments which differed by antineoplastic type and administration method, including fulvestrant (injection, hormonal), letrozole (oral, hormonal), capecitabine (oral, chemotherapy), and paclitaxel (infusion, chemotherapy).
Results: Average total time spent on health care ranged from 7% to 10% of all days included within the initial 3 months of treatment, depending on treatment. The greatest time contributions were time spent traveling for care and on inpatient services. Time with providers contributed modestly to total care time. Patients receiving infusion/injection treatments, compared with those receiving oral therapy, spent more time in ambulatory care. Health care time was higher for patients receiving chemotherapeutic agents compared to those receiving hormonal agents.
Conclusion: Time spent traveling and receiving inpatient care represented a substantial burden to patients with MBC, with variation in time by treatment type and administration method.
Keywords: indirect costs; metastatic breast cancer; patient time.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Rocque received research funding from Pfizer, and Carevive and consulting fees and travel from Genentech and Pfizer unrelated to this manuscript. Dr. Rocque is also supported by an American Cancer Society Mentored Research Scholar Grant (MRSG‐17‐051‐01‐PCSM). No other authors have conflicts to declare.
Figures
References
-
- Zafar SY. Financial toxicity of cancer care: it's time to intervene. J Natl Cancer Inst. 2016;108:djv370. - PubMed
-
- Nardi EA, Wolfson JA, Rosen ST, et al. Value, access, and cost of cancer care delivery at academic cancer centers. J Natl Compr Canc Netw. 2016;14:837‐847. - PubMed
-
- Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology Value Framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34:2925‐2934. - PubMed
-
- Seabury SA, Frasco MA, van Eijndhoven E, Sison S, Zacker C. The impact of self‐ and physician‐administered cancer treatment on work productivity and healthcare utilization. Res Social Adm Pharm. 2018;14:434‐440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical